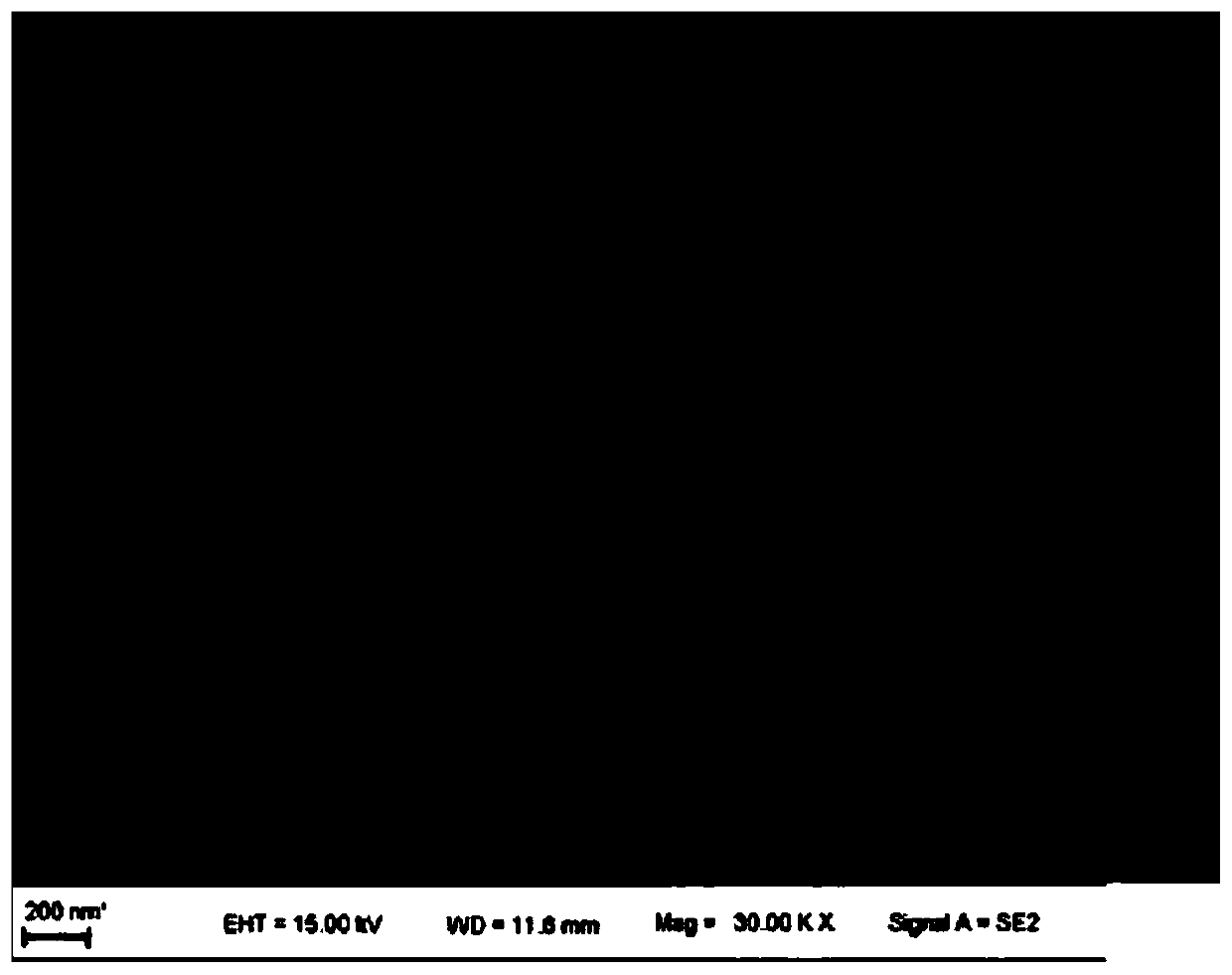
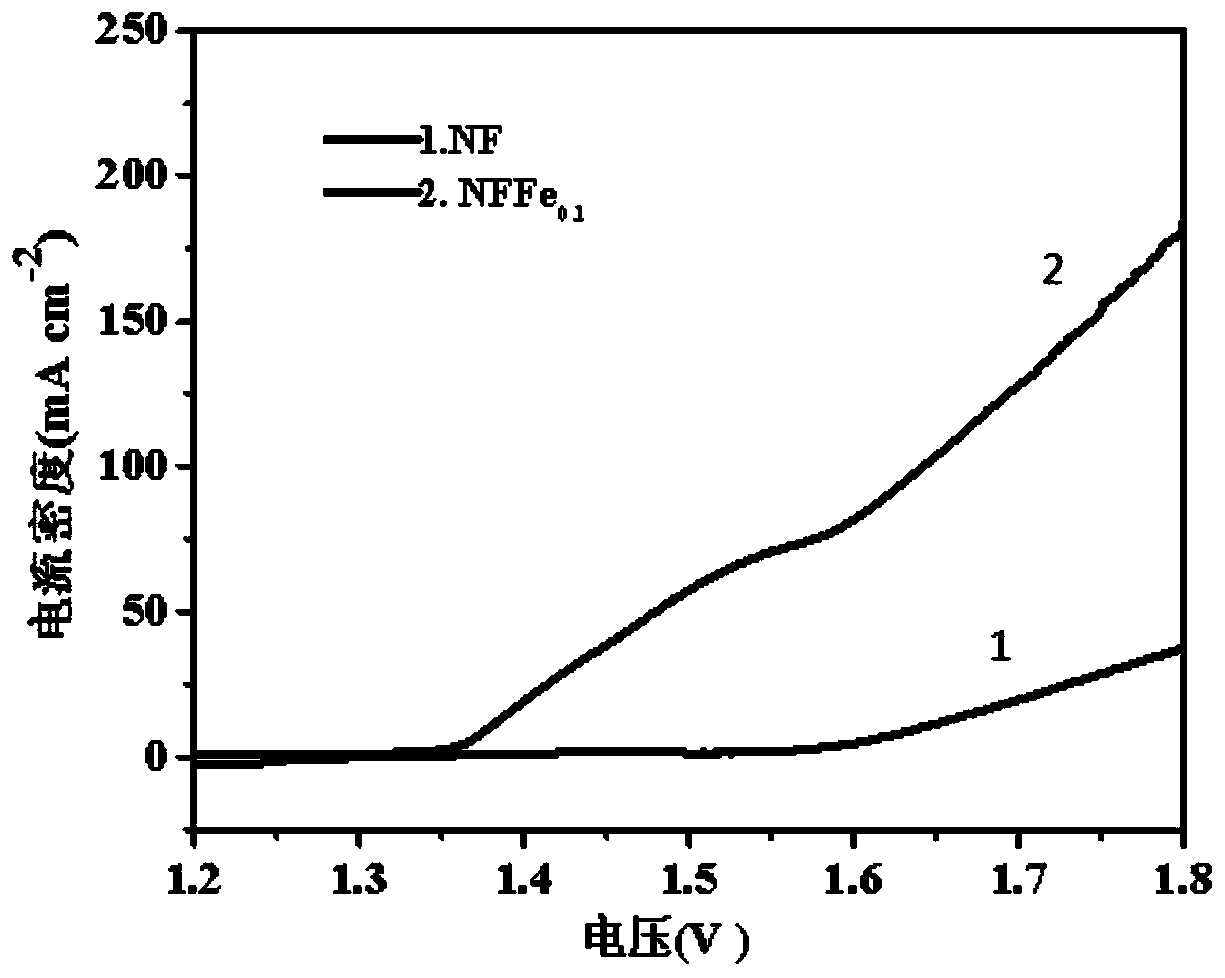
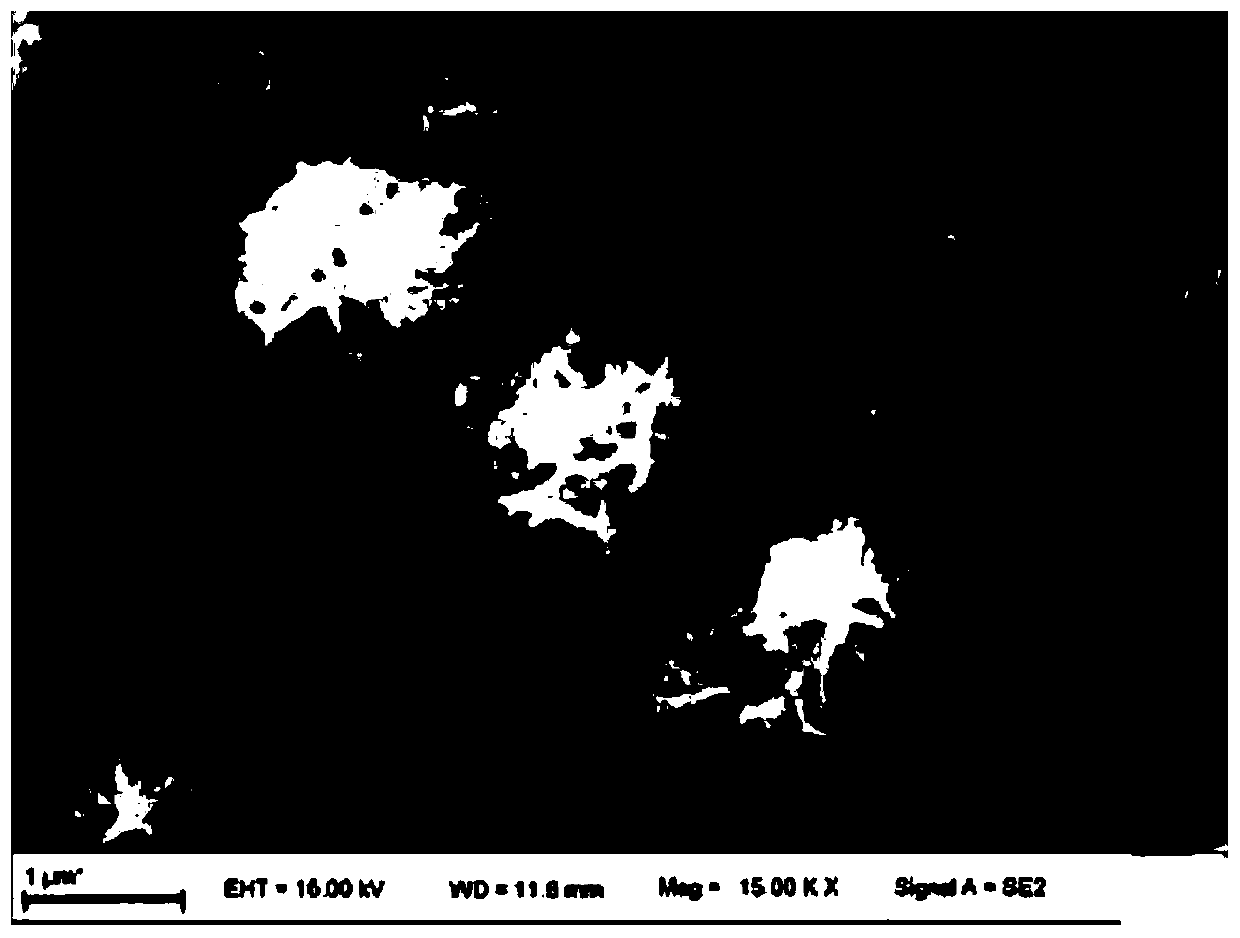
Preparation method and application of self-supporting controllable electrode material for oxygen evolution
An electrode material, oxygen technology, applied in the direction of electrodes, electrode shapes/types, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A method for preparing a self-supporting controllable electrode material for oxygen evolution, comprising the following steps: x=0.1.
[0032] 0.1mmol of FeCl 3 ·6H 2 O is fully dissolved in 30mL of deionized water and transferred to a Teflon-lined container, and the above solution is added to the container of solution A as a titration solution through a conductive titration head, and the two are mixed evenly, and at the same time, the conductive drop An electric field was applied between the head and the solution, and the voltage was set at +1000V, and the nickel foam substrate that had been ultrasonically cleaned in hydrochloric acid, ethanol and deionized (DI) water was immersed in sequence, and heated at 120° C. for 3 h. After cooling to room temperature, the obtained Ni foam was washed with water three times and dried at 60 °C for 2 hours to obtain the as-prepared electrode material.
[0033] A three-electrode system was used to test the electrocatalytic oxygen e...
Embodiment 2
[0037] A method for preparing a self-supporting controllable electrode material for oxygen evolution and the method includes the following steps: x=0.2
[0038] 0.2mmol of Fe(NO 3 ) 3 After being fully dissolved in 30mL of deionized water, it was transferred to a Teflon-lined container, and the above solution was added to the container of Solution A as a titration solution through a conductive titration head, and after the two were mixed evenly, and at the same time, the Apply an electric field to the solution, set the voltage to +500V, immerse the nickel foam substrate that has been ultrasonically cleaned in hydrochloric acid, ethanol and deionized (DI) water in sequence, and heat at 120°C for 2h. After cooling to room temperature, the obtained Ni foam was washed with water three times and dried at 60 °C for 2 hours to obtain the as-prepared electrode material.
[0039] A three-electrode system was used to test the electrocatalytic oxygen evolution performance of the obtain...
Embodiment 3
[0042] A method for preparing a self-supporting controllable electrode material for oxygen evolution and the method includes the following steps: x=0.3
[0043] Fully dissolve 0.3mmol of ferric acetate in 30mL of deionized water and transfer it to a Teflon-lined container. Add the above solution as a titration solution to the container of solution A through a conductive titration head, mix the two evenly, and simultaneously Apply an electric field between the conductive dropper and the solution, set the voltage to -500V, and immerse the nickel foam substrate that has been ultrasonically cleaned in hydrochloric acid, ethanol and deionized (DI) water successively, and heat at 120°C for 2h . After cooling to room temperature, the obtained Ni foam was washed with water three times and dried at 60 °C for 2 hours to obtain the as-prepared electrode material.
[0044] A three-electrode system was used to test the electrocatalytic oxygen evolution performance of the obtained self-sup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com