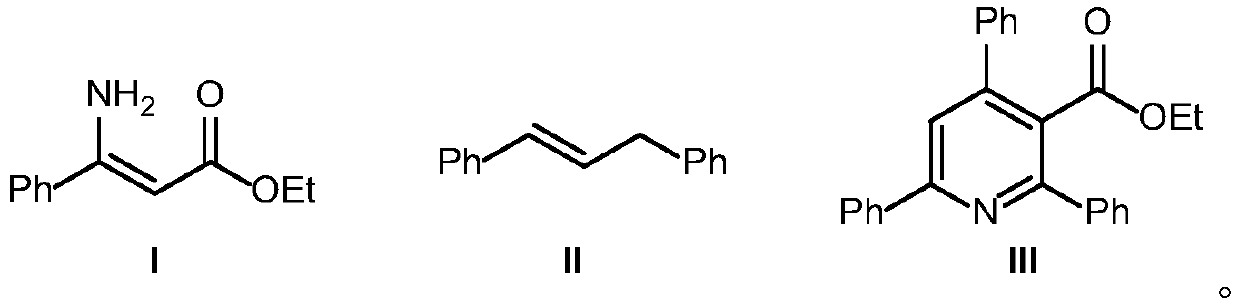
Synthetic method of ethyl 2,4,6-triphenylnicotinate
A technique of triphenylnicotinic acid ethyl ester and a synthetic method, applied in the direction of organic chemistry, etc., can solve the problems of difficult substrates and cumbersome operations, and achieve the effects of easy-to-obtain, simple raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 10mL round bottom flask, add 0.24mmol (0.047g) (E)-1,3-diphenylpropene, 0.24mmol (0.054g) DDQ, 3mL1,4-dioxane, and stir at room temperature for 10 minutes , add 0.2mmol (0.038g) (E)-3-phenyl-3-aminoacrylic acid ethyl ester, react for 2 hours, then add 0.42mmol (0.095g) DDQ, continue to react for 0.5 hours, obtain the reaction solution and remove the solvent under reduced pressure , separated by column (200-300 mesh), using petroleum ether: ethyl acetate = 20:1 (volume ratio) as eluent, to obtain colorless oily ethyl 2,4,6-triphenylnicotinate ( 0.061g), the yield was 80%. 1 H NMR (500MHz, CDCl 3 ):8.19-8.17(m,2H),7.81-7.79(m,2H),7.77(s,1H),7.54-7.46(m,11H),4.02(q,J=7.1Hz,2H),0.93( t,J=7.1Hz,3H). 13 C NMR (125MHz, CDCl 3 ):168.7,157.2,156.6,149.6,140.0,138.6,138.5,129.5,128.7,128.6,128.5,128.2,128.1,127.2,126.8,119.4,61.3,13.4.ESI m / z[M+H] + 380.2.
Embodiment 2
[0025] In a 10mL round bottom flask, add 0.24mmol (0.047g) (E)-1,3-diphenylpropene, 0.2mmol (0.045g) DDQ, 3mL 1,4-dioxane, and stir at room temperature for 10 minutes Afterwards, add 0.2mmol (0.038g) (E)-3-phenyl-3-aminoacrylic acid ethyl ester, react for 2 hours, then add 0.4mmol (0.091g) DDQ, continue to react for 0.5 hours, and remove the reaction solution under reduced pressure Solvent, separated by column (200-300 mesh), using petroleum ether: ethyl acetate = 20:1 (volume ratio) as eluent, to obtain colorless oily ethyl 2,4,6-triphenylnicotinate (0.056 g), yield 74%.
Embodiment 3
[0027] In a 10mL round bottom flask, add 0.24mmol (0.047g) (E)-1,3-diphenylpropene, 0.24mmol (0.054g) DDQ, 4mL 1,4-dioxane, and stir at room temperature for 10 minutes After that, add 0.2mmol (0.038g) (E)-3-phenyl-3-aminoacrylic acid ethyl ester, react for 2 hours, then add 0.44mmol (0.10g) DDQ, continue to react for 0.5 hours, and remove the reaction solution under reduced pressure Solvent, separated by column (200-300 mesh), using petroleum ether: ethyl acetate = 20:1 (volume ratio) as eluent, to obtain colorless oily ethyl 2,4,6-triphenylnicotinate (0.055 g), yield 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com