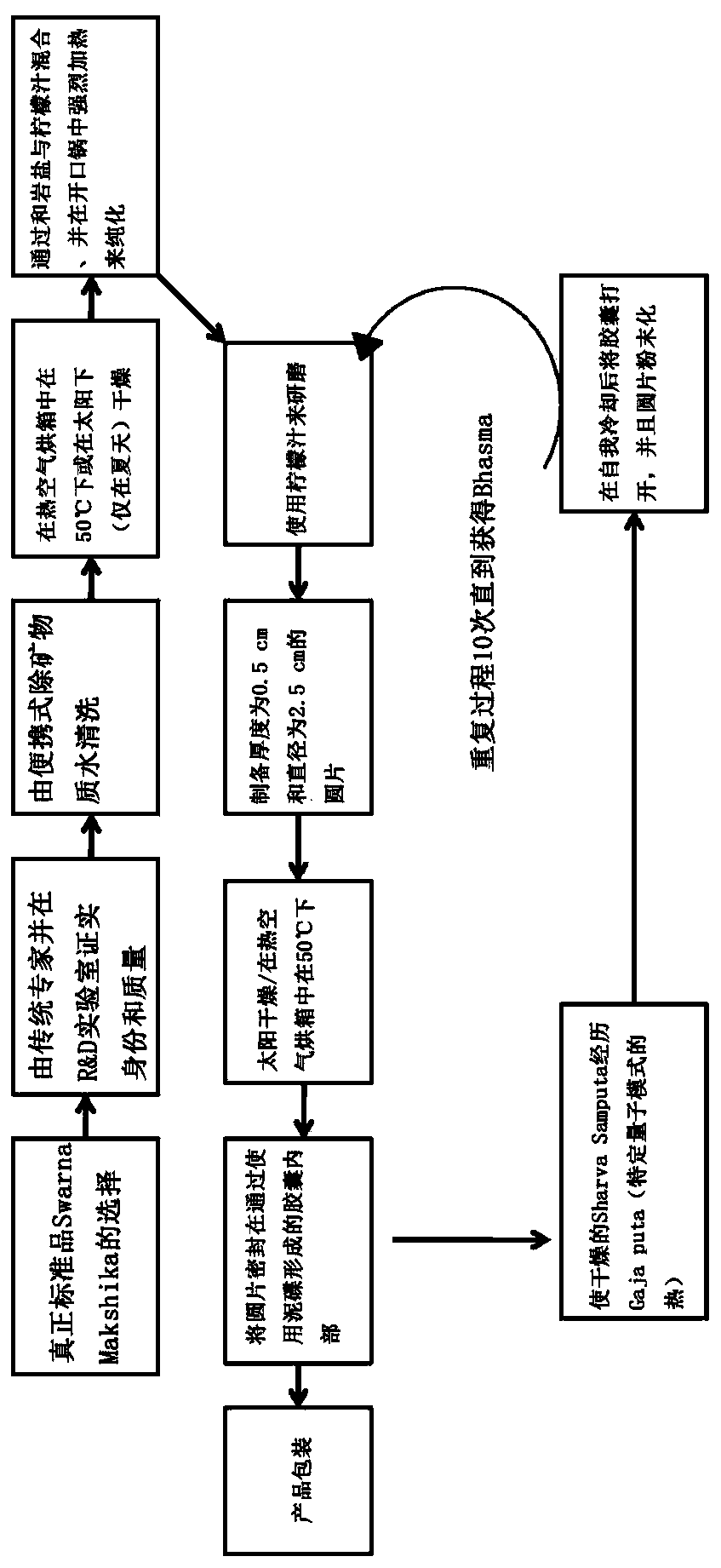
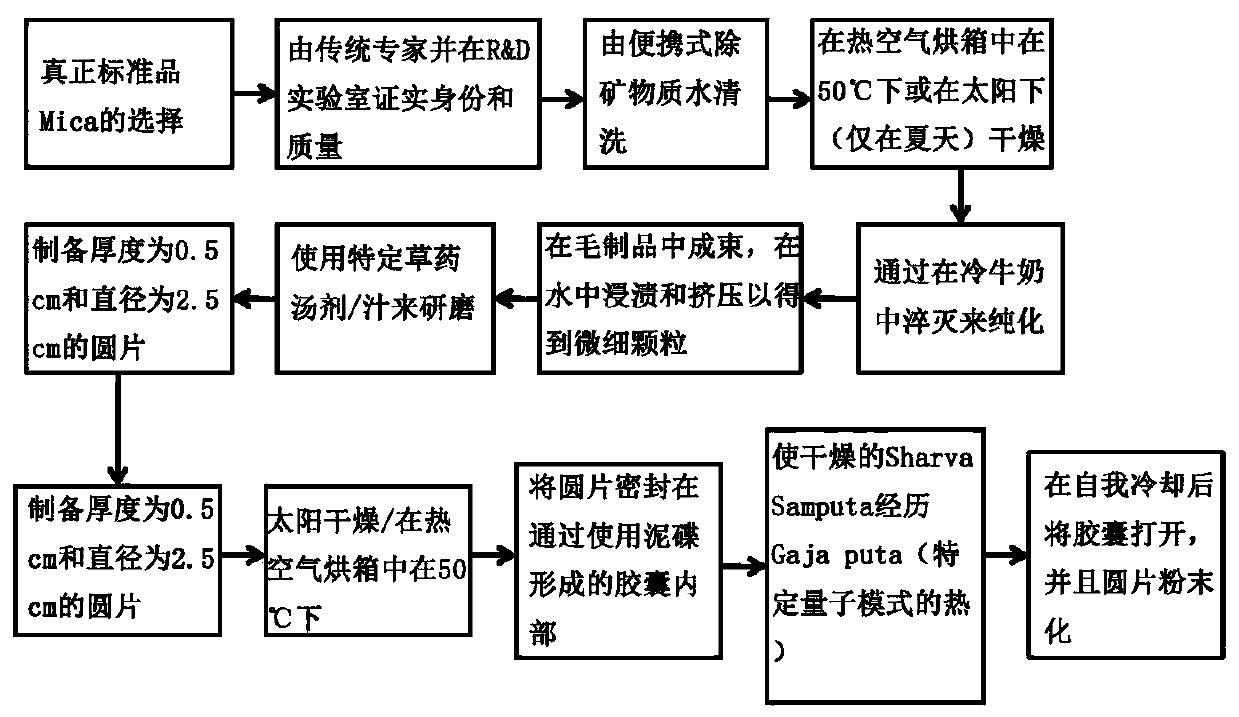
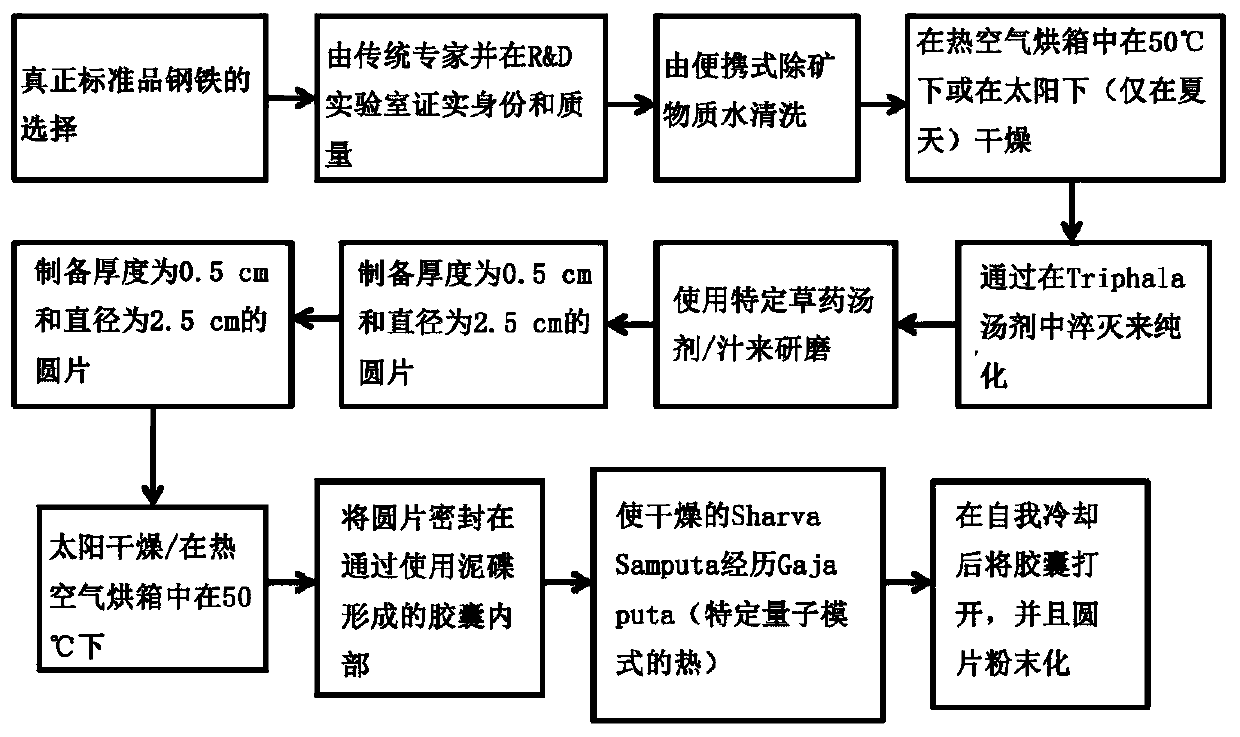
Herbo-mineral formulation for prevention, treatment and management of diabetes and method of preparation thereof
A diabetes and mineral technology, applied in the direction of drug combination, pharmaceutical formula, non-living material medical raw materials, etc., can solve the problems of controversial effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Embodiment 1: experimental research
[0108] The purpose of this study was to analyze the effects of the test product on pancreatic blood glucose, lipids, histone, pancreatic amylase and islet cells in alloxan-induced diabetic rats.
[0109] Experiment details:
[0110] Animals: Albino rats of either sex, Wistar strain, were fed standard rat pellets and water ad libitum.
[0111] Experimental diabetes: Diabetes was induced by a single intraperitoneal injection of 5% alloxan monohydrate dissolved in 0.9% saline at a dose of 200 mg / kg body weight after a 16-hour fast.
[0112] Test Drug Treatment: Tablets were crushed, weighed and mixed with water to form a homogeneous suspension, administered orally every morning using a gastric tube.
[0113] Dose: 1ml (30mg / cc)
[0114] result:
[0115] image 3 Shown are the results of oral glucose tolerance tests in normal and diabetic rats after test product treatment. Treated normal rats showed a hypoglycemic effect, whil...
Embodiment 2
[0123] Embodiment 2: clinical research
[0124] The purpose of this clinical study is to evaluate the clinical efficacy and safety (long and short term) of the test product as monotherapy and as an adjunct to other oral hypoglycemic agents in the management of NIDDM. The study helps evaluate the effect of the test product on the clinical and biochemical characteristics of diabetic patients.
[0125] Study design: This is a prospective, open label, nonrandomized, phase III clinical trial conducted at the Out Patient Department, Muniyal Ayurvedic Hospital and Research Center, Manipal, India.
[0126] Materials and methods
[0127] Inclusion criteria: A total of 340 sexes, patients diagnosed with NIDDM between the ages of 30 and 60, and patients willing to give informed consent were included in this study. The WHO diagnostic criteria (1980) are considered for the diagnosis of NIDDM (for newly diagnosed patients: FBSL >120 mg%, and after consuming 75 g of glucose 2 hours: >180 m...
Embodiment 3
[0144] Example 3: Quality of Life Study
[0145] Study Design: Prospective observational study design Data Sources:
[0146] Main data: Patient case records and interviews with patients. Secondary data: Internet sites, Ayurveda textbooks, periodicals.
[0147] standard constrain:
[0148] all type 2 diabetics
[0149] Age 18-75
[0150] Diabetics taking only test product tablets
[0151] Patients who agree to sign the ICF
[0152] Patients whose treatment pattern does not change up to 3 months
[0153] Exclusion criteria:
[0154] Type I diabetics.
[0155] pregnant lady.
[0156] lactating women.
[0157] chronically ill patients.
[0158] Patients undergoing lifestyle changes or diet therapy (prediabetic patients).
[0159] Patients who refused to participate in the study.
[0160] Study setting: This study was conducted in a group of patients who received tablets of the test product for the treatment of type II diabetes in and around Udupi and Manipal distric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com