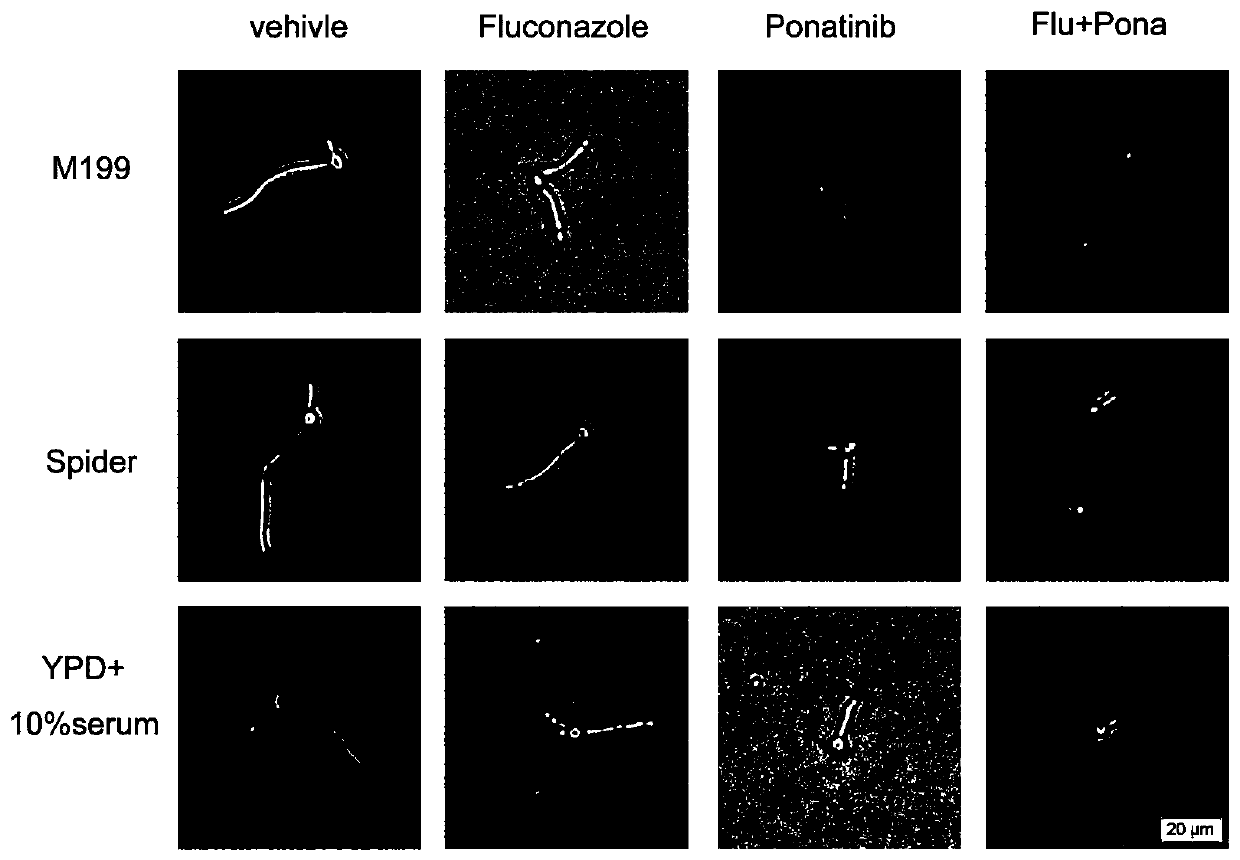
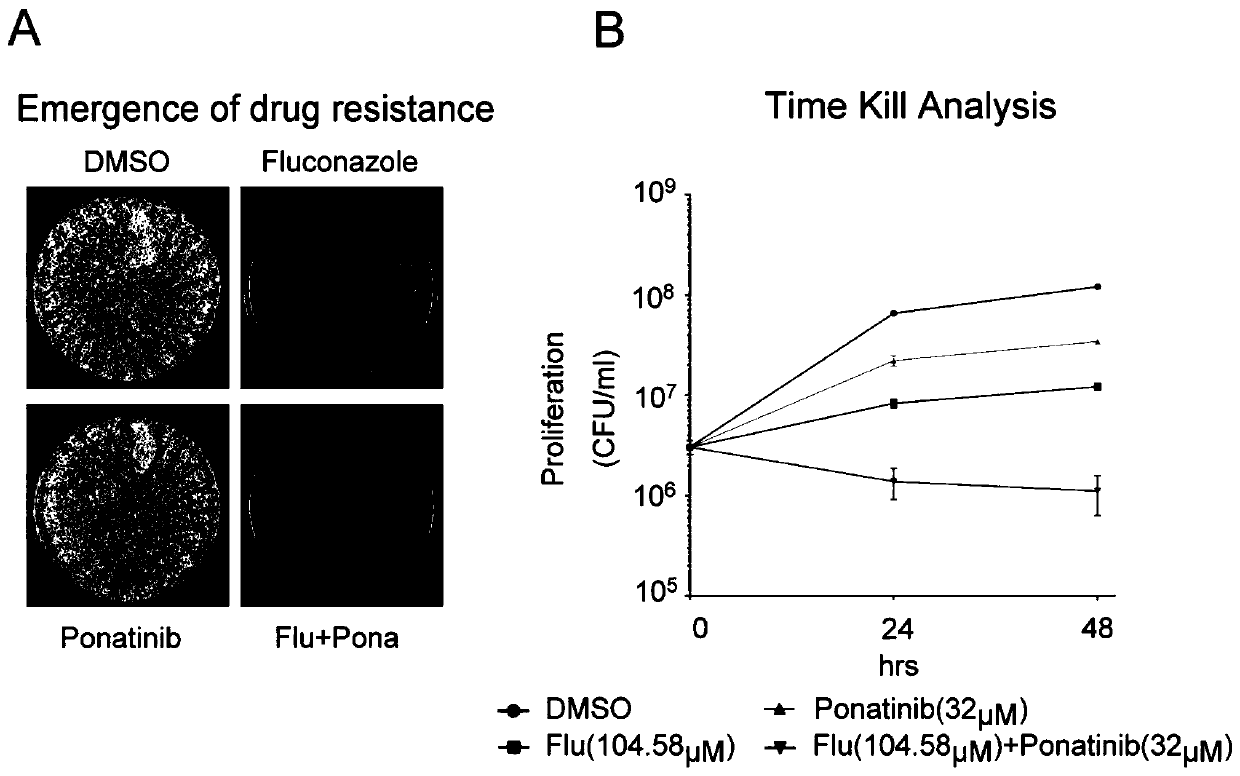
Application of Ponatinib in preparing drugs for treating fungal infections
A technology of fungal infection and ponatinib, applied in the direction of antifungal agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., to achieve the effects of increasing accumulation, promoting killing activity, and good killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Inhibitory effect of ponatinib and fluconazole on the growth of Candida albicans cells and mycelia
[0033] Experimental process: In a 96-well plate, prepare doubly diluted fluconazole along the Y axis and doubly diluted ponatinib along the X axis, with a final volume of 100 μl per well, and then place the white rosary recovered from -80°C Bacterial suspension (OD 600 =0.02), then inoculated in a 96-well plate containing drugs (100 μl / well), incubated at 30°C, and detected OD with a microplate reader after 16 hours 600 . The FICI of a drug combination is determined from a single FIC value (FIC=[x] / MICx, where [x] is the minimum inhibitory concentration of the drug in combination, FICI=FIC ponatinib +FIC fluconazole ).
[0034]The minimum inhibitory concentration (Minimal Inhibitory Concentration, MIC) of fluconazole against Candida albicans was 1.63 μM, and the MIC of ponatinib against Candida albicans was 4 μM. Natinib increased the sensitivity of Candida albicans...
Embodiment 2
[0039] Inhibition of ponatinib and fluconazole against other fungi (Saccharomyces cerevisiae, Cryptococcus neoformans, Aspergillus fumigatus)
[0040] Experimental process: In a 96-well plate, prepare doubly diluted fluconazole or voriconazole along the Y axis and doubly diluted ponatinib along the X axis, with a final volume of 100 μl per well, and then place the recovered from -80°C Bacterial suspension (OD 600 or OD 530 =0.02), then inoculated in a 96-well plate containing drugs (100 μl / well), incubated at 30°C, and detected OD with a microplate reader after 16 hours 600 or OD 530 . The FICI of a drug combination is determined from a single FIC value (FIC=[x] / MICx, where [x] is the minimum inhibitory concentration of the drug in combination, FICI=FIC ponatinib +FIC antifungal ).
[0041] The MIC of fluconazole on Saccharomyces cerevisiae was 52.29 μM, and when Ponatinib was used alone, the inhibitory effect on Saccharomyces cerevisiae was not significant, but when flu...
Embodiment 3
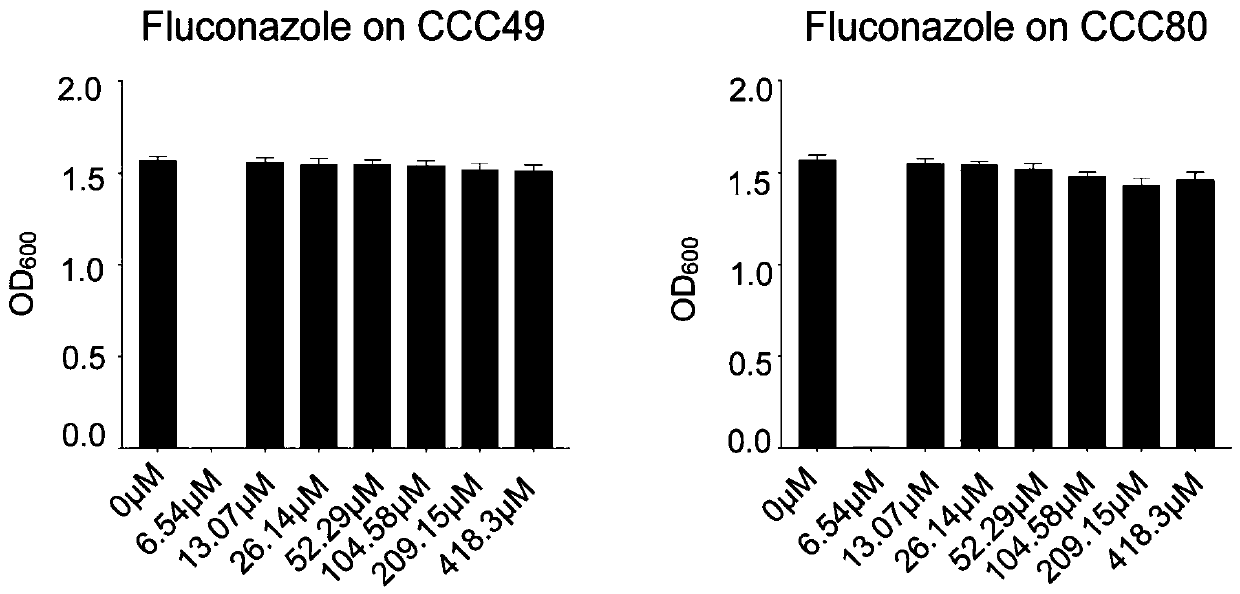
[0051] Bacteriostatic effect of ponatinib and fluconazole on clinical fluconazole-resistant strains
[0052] Experimental process: In a 96-well plate, prepare doubly diluted fluconazole along the X axis, with a final volume of 100 μl per well, and then make a bacterial suspension (OD 600 =0.02), then inoculated in a 96-well plate containing drugs (100 μl / well), incubated at 30°C, and detected OD with a microplate reader after 16 hours 600 .
[0053] The MICs of fluconazole against FLC-resistant Candida albicans CCC49 and CCC80 were 104.58 μM and 52.29 μM, respectively. Fluconazole was applied to clinical fluconazole-resistant strains with a certain concentration gradient, and incubated at 30°C for 16 hours. Detect OD 600 , and finally make a histogram, such as figure 2 shown;
[0054] Experimental process: In a 96-well plate, prepare doubly diluted fluconazole along the Y-axis and doubly diluted ponatinib along the X-axis, with a final volume of 100 μl per well, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com