Anemarrhena asphodeloides bge steroid saponin compound, and preparation method and application thereof
A technology for steroidal saponins and compounds, applied in the field of medicine, can solve problems such as patents or literature reports that have not yet been found, and achieve the effects of significant anti-tumor activity, simple extraction and separation methods, and novel structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
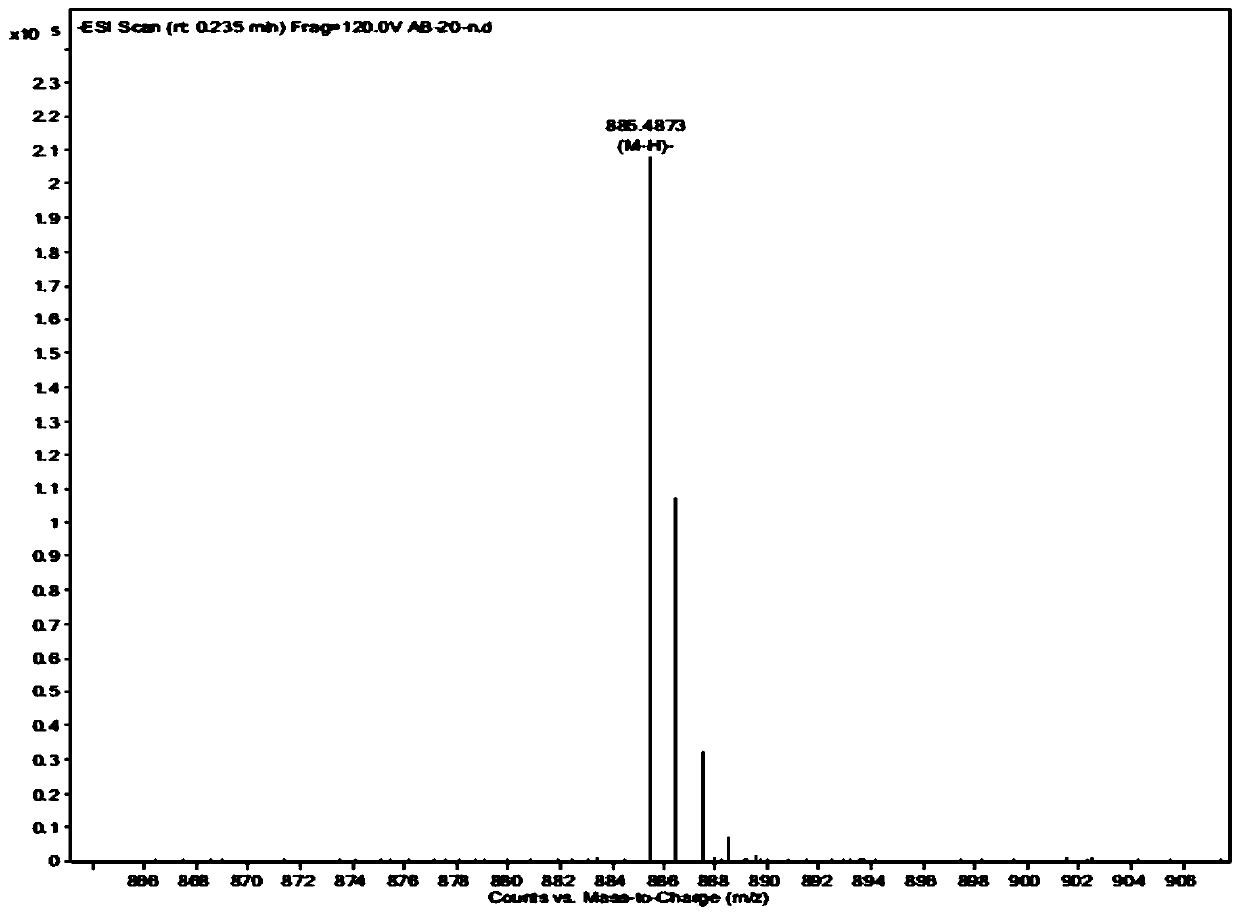
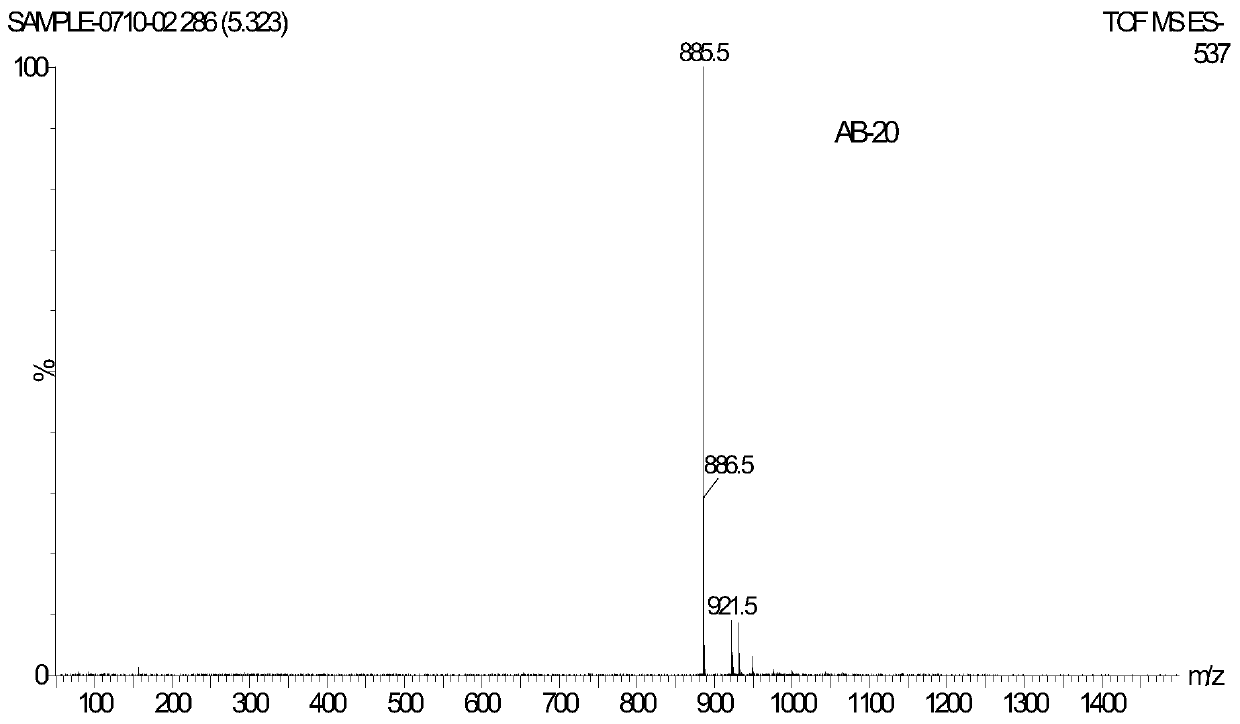
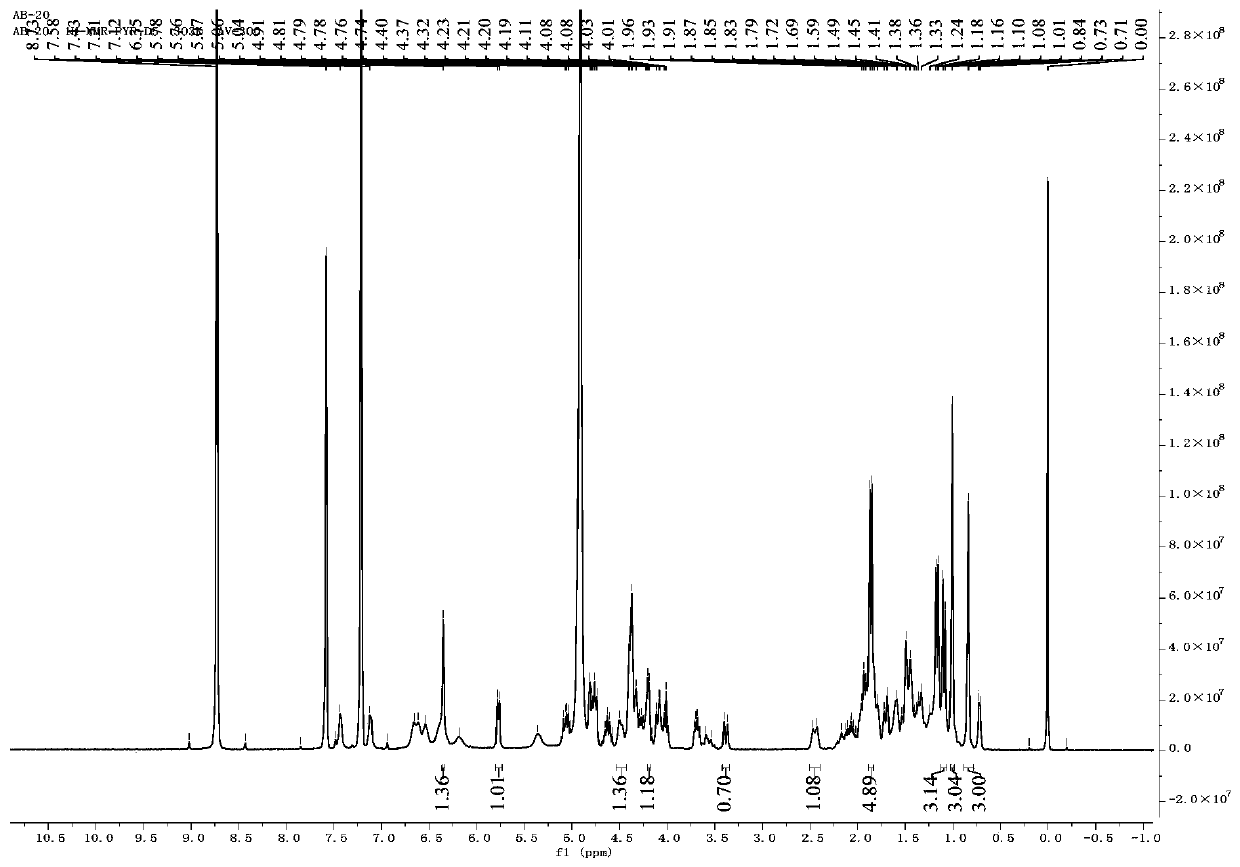
[0035] Preparation of steroidal saponins
[0036] Weigh 10kg of dried rhizomes of Anemarrhena vulgaris and place them in an extraction tank. Add 50L of 75% ethanol. After soaking overnight, heat in a water bath to reflux and extract for 3 hours. Filter, and extract the filter residue twice with 75% ethanol (50L), each for 3 hours, and combine three times The filtrate was concentrated to obtain an extract-like ethanol extract (3.09kg). The ethanol extract is suspended in purified water to obtain a suspension, and then extracted with an equal volume of petroleum ether, ethyl acetate, and water-saturated n-butanol three times in sequence, and the extract is concentrated to obtain an extract of three parts. The n-butanol extract (600g) was roughly divided by macroporous adsorption resin, and the first gradient elution was carried out with 10%, 30%, 50%, 70%, 95% (v / v) ethanol-water. The eluate from the 70% ethanol fraction was crudely separated by silica gel column chromatography. ...
Embodiment 2
[0050] Anti-tumor activity test of compound I
[0051] 1. Cell lines and reagent materials Human breast cancer cell line MCF-7 and human liver cancer cell line HepG2 were provided by the American Type Culture Collection (ATCC); DMEM medium, RPMI-1640 medium, fetal bovine serum, trypsin, DMSO And MTT etc.
[0052] 2. MTT method to detect cell survival rate
[0053] Culture the MCF-7 and HepG2 cell lines with RPMI-1640 medium containing 10% fetal bovine serum, and take the MCF-7 and HepG2 cell lines that are in the logarithmic growth phase and are in good condition. The medium is discarded and pre-cooled aseptically Wash three times with PBS, then digest with trypsin solution and observe with an inverted biological microscope. After digestion, the cells are mixed evenly. Spread the MCF-7 and HepG2 cell lines on a 96-well plate respectively to ensure that the MCF-7 cell line is 2500 cells / well and the HepG2 cell line is 2000 cells / well. The drug is administered at constant temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com