Antituberculous CTL epitope peptide and application thereof
A tuberculosis and drug technology, applied in the field of anti-tuberculosis CTL epitope peptides, can solve the problems of large differences in the protective effect of adults
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
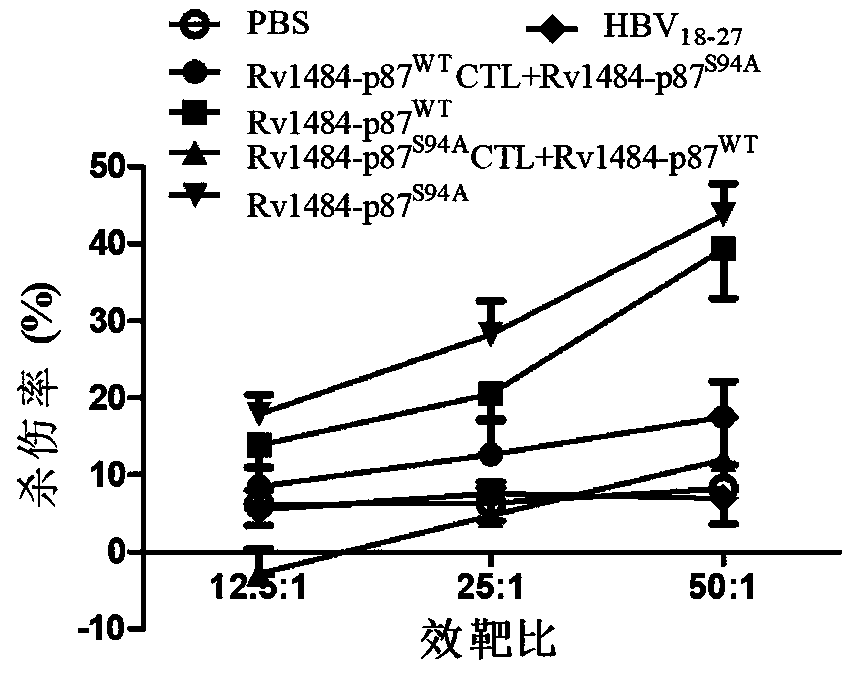
Embodiment 1
[0021] Embodiment 1 binding force and stability experiment
[0022] The binding force experiment scheme is as follows;
[0023] (1) Dissolve the prepared candidate peptides in sterilized PBS (pH 7.2), and make 1 mg / mL aliquots for use;
[0024] (2) T2A2 cells in good growth state were obtained, centrifuged at 2000 rpm at 4°C for 5 min, and washed twice with serum-free IMDM medium. Adjust density to 1×10 6 cells / mL, spread in 24-well plate, 500 μL / well.
[0025] (3) Take out the dissolved and subpackaged peptides in advance and put them in a 4°C refrigerator. After the cells are plated, add human β 2 Microglobulin (β 2 -M) (0.5 μg / mL), and then add the dissolved antigen peptide (50 μg / mL). According to the experimental arrangement, set up: experimental group (epitope peptide), negative control group (PBS), positive control group (COX-2 321-329 ) and the background group (T2A2 cells). Mix the cell suspension with pinch plate, put it in the cell culture incubator at 37°C,...
Embodiment 2
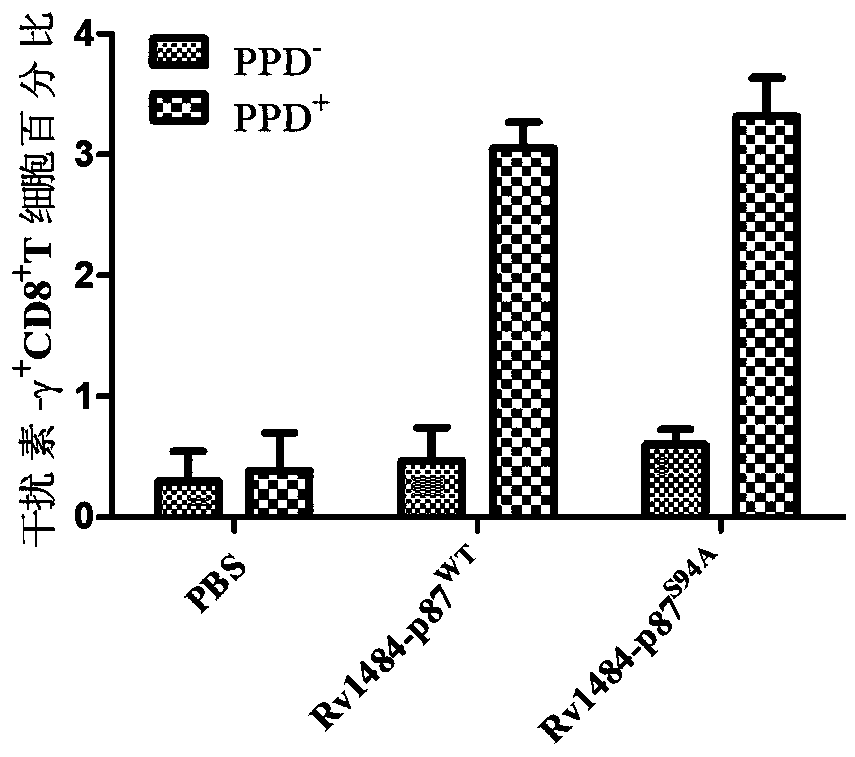
[0048] Example 2 Separation and induction of human peripheral blood mononuclear cells (PBMCs)
[0049] (1) According to the amount of 30U heparin sodium / mL peripheral blood, add a certain amount of heparin sodium into a 40mL centrifuge tube according to the amount of peripheral blood drawn. First recruited volunteers were tested for peripheral blood HLA type, and some HLA-A2+ volunteers underwent tuberculin (PPD) intradermal test. 40 mL of peripheral blood was drawn from each volunteer obtained from the screening. The blood in the syringe should be injected into the wall when it is transferred into a 50mL centrifuge tube, then shake it slowly several times and place it on ice.
[0050] (2) Dilute the retrieved anticoagulated peripheral blood with PBS of pH 7.2 at a ratio of 1:1, and gently pipette to mix. Take 10 15mL centrifuge tubes, open the human peripheral blood lymphocyte separation medium in the ultra-clean workbench, and add 4mL of the separation medium to each 15mL ...
Embodiment 3
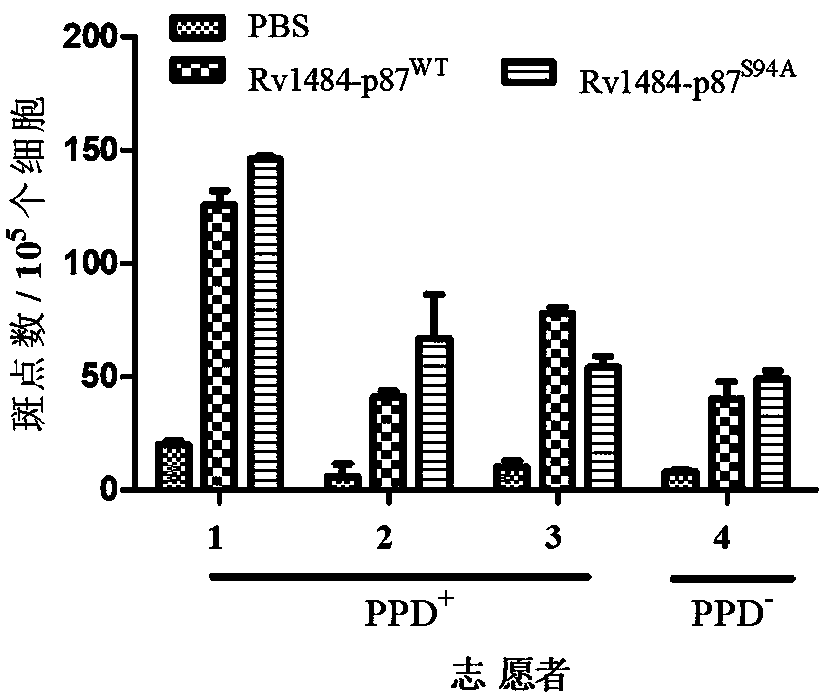
[0053] Embodiment 3 ELISPOT experiment (enzyme-linked immunospot experiment)
[0054] ELISPOT experiments were performed using human IFN-γ pre-coating kits.
[0055] (1) Collection of target cells: T2A2 cells in culture were observed under an inverted optical microscope, and cells in a better state were selected. Gently blow and aspirate the medium in the culture flask with an electric pipette to make a uniform cell suspension. Transfer to a 15mL centrifuge tube, centrifuge in a horizontal centrifuge: 1000rpm, 8min. After discarding the supernatant, add serum-free IMDM medium and wash once. Adjust the cell density to 2 x 10 6 cells / mL, spread into a 24-well plate, 1 mL / well. Set epitope peptide group, PBS group, blank group.
[0056] (2) Target cell-loaded peptide: add epitope peptide / PBS (pH 7.2) to the cell suspension in each well at an amount of 50 μg / mL, and add human β at an amount of 3 μg / mL 2 -M. Gently blow the cells in the well plate with a pipette, and incubat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com