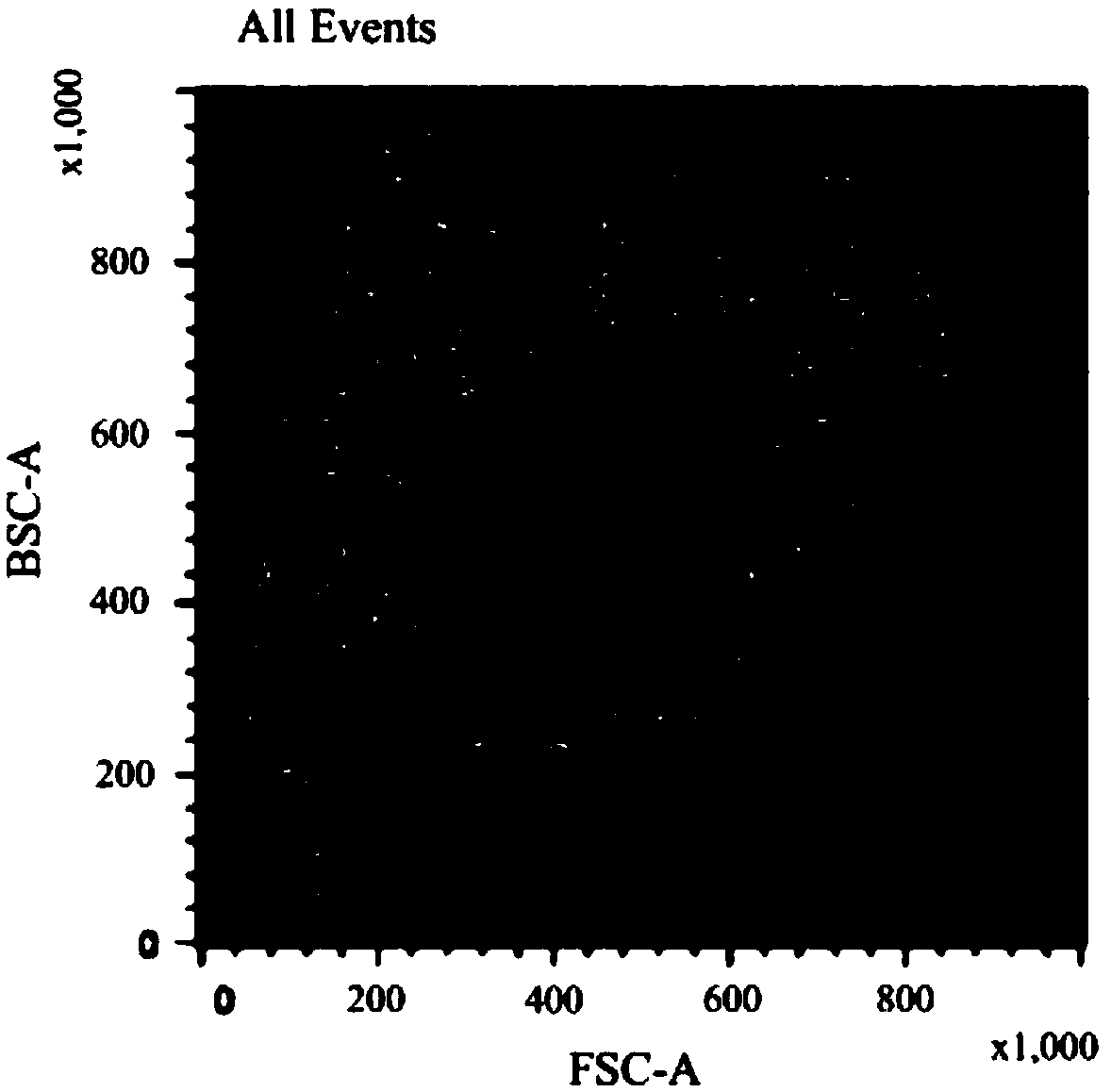
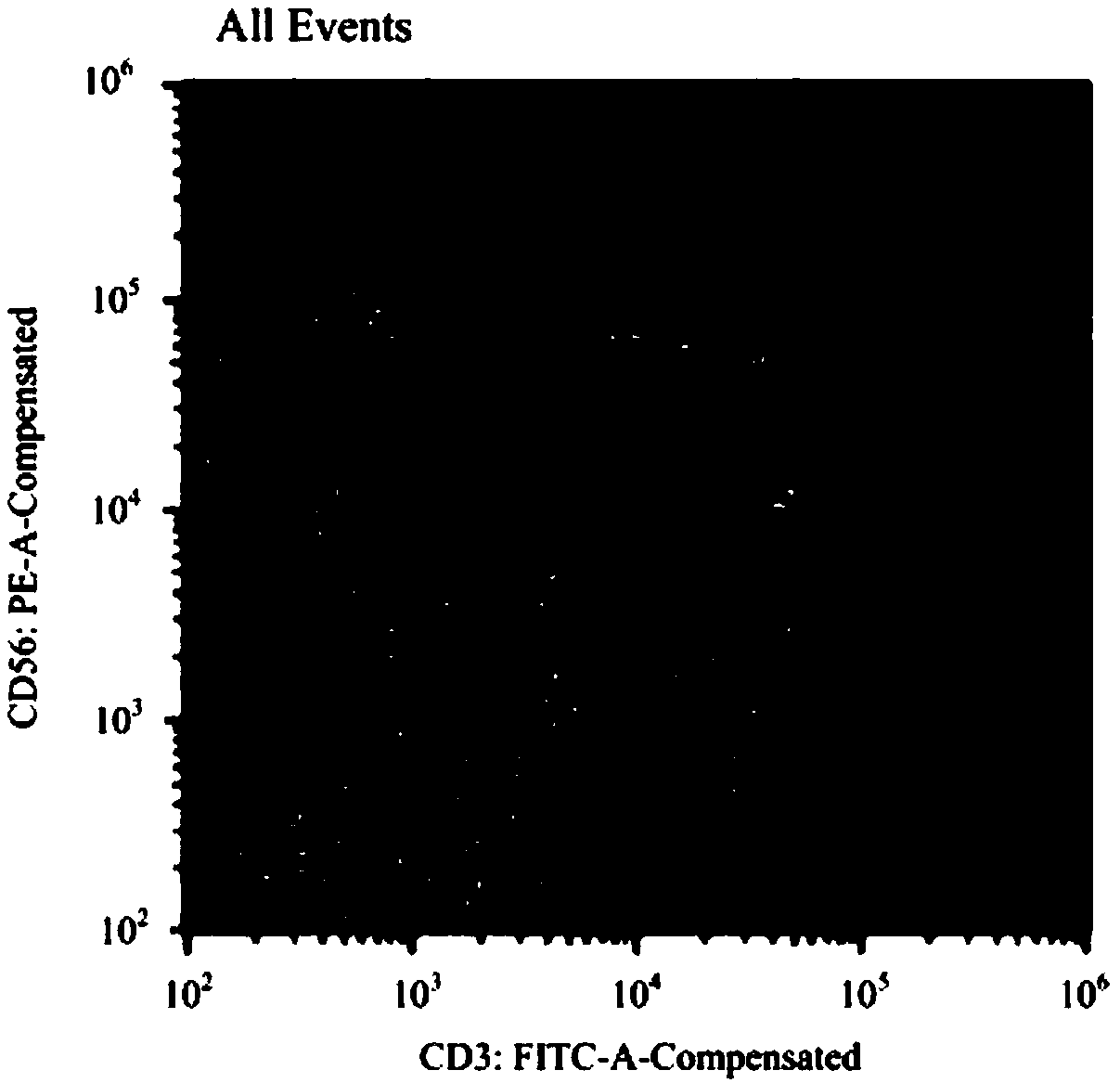
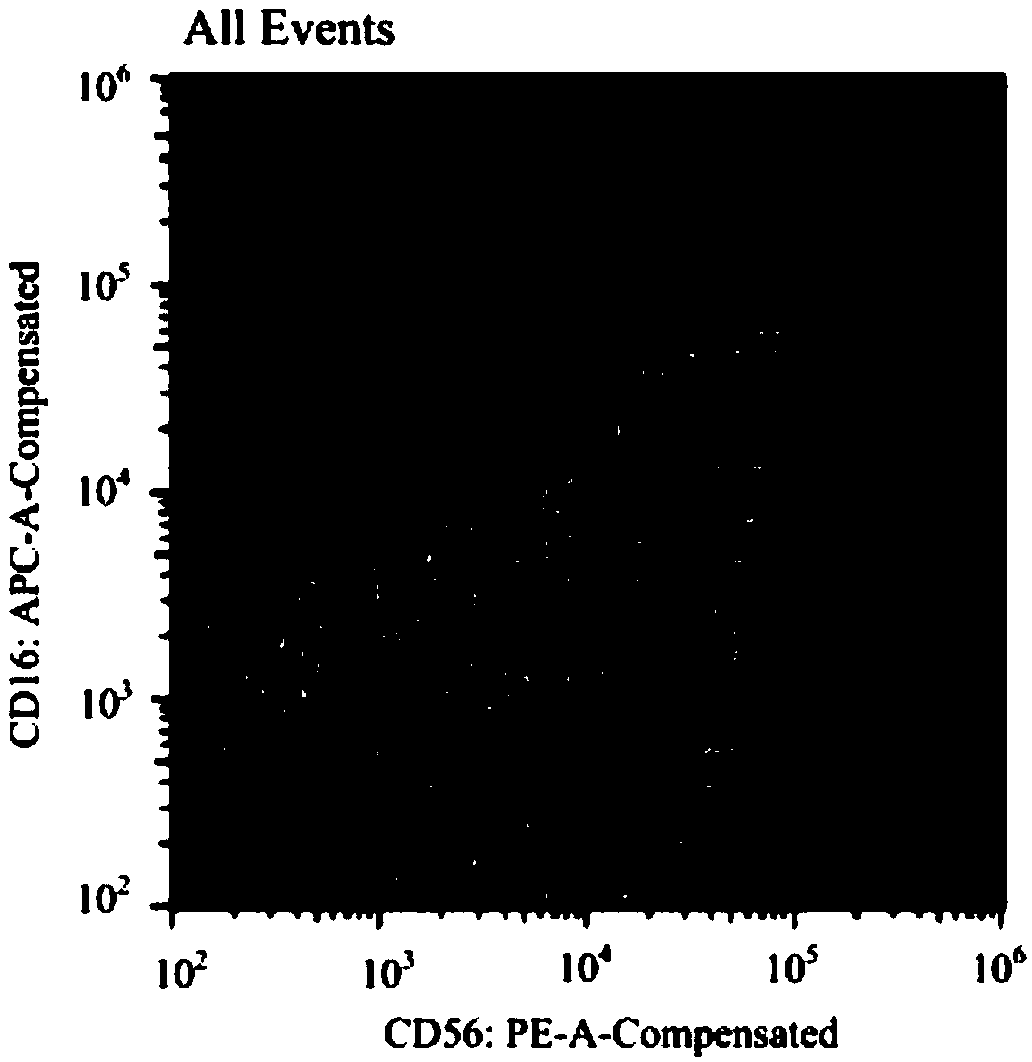
Serum-free cell culture solution for in-vitro amplification of nature killer cells and nature killer T cells
A technology of natural killer cells and cell culture, which is applied in the direction of cell culture active agents, tissue culture, animal cells, etc., and can solve the problems of cells being limited to the use of hosts and cell recipient rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the test of serum-free culture fluid
[0051] The purpose of this example is to establish a serum-free cell culture medium that can be used to expand natural killer cells (NK cells) / natural killer T cells (NKT cells) in vitro, and this cell culture medium does not need to add additional autologous plasma or peripheral blood samples. Serum, or other animal-derived serum; peripheral blood mononuclear cells were obtained as described above, and the peripheral blood samples were obtained from autologous serum, SILAC Advanced DMEM / F-12, UltraGRO TM Or RPMI-1640 medium four kinds of cell culture medium for culture, wherein, the autologous serum of the source of the peripheral blood sample contains 500IU / ml rhIL-2 and 50ng / ml Anti-CD3 monoclonal antibody, SILAC Advanced DMEM / F -12. UltraGRO TM and RPMI-1640 medium respectively contained 500IU / ml of rhIL-2, 50ng / ml of Anti-CD3 monoclonal antibody and 2U / ml of heparin. After culturing, the cell density was evaluat...
Embodiment 2
[0055] Embodiment 2, optimization of initial cell number
[0056] Continuing from the above, this example continues to discuss the optimal initial cell density when expanding NK / NKT cells with serum-free cell culture medium, which obtains peripheral blood mononuclear cells as described above, and uses UltraGRO TM As a basal medium, add 500IU / ml rhIL-2, 50ng / ml Anti-CD3 monoclonal antibody and 2U / ml heparin for culture, and then evaluate the cell density by the aforementioned cell counting method.
[0057] As shown in Table 2, the initial cell density was 1.00x10 6 , 2.00x10 6 , 3.00x10 6 and 5.00x10 6 cells / ml was cultured, and the cells were taken out from the culture plate on the 5th, 7th, 9th, 12th, 14th, 16th, 18th and 20th day after culture for counting, and the cell density was readjusted to 1.00x106cells / ml, the results can be It is known that when NK / NKT cells are expanded in vitro with these initial cell densities, the cell survival rate can be maintained above 90...
Embodiment 3
[0060] Embodiment 3, optimization of heparin (Heparin) concentration in serum-free cell culture medium
[0061]In one embodiment of the present invention, heparin is used as part of the role of replacing serum. Therefore, this embodiment continues to discuss. Under the culture conditions of serum-free cell culture medium and initial cell density established in Examples 1 and 2, Can further enhance the heparin concentration of cell growth efficiency; It obtains the aforementioned peripheral blood mononuclear cells, with a preferred embodiment 2.00×10 6 cells / mL is the initial cell density, using UltraGRO TM The cell culture medium (containing 500 IU / ml of rhIL-2 and 50 ng / ml of Anti-CD3 monoclonal antibody) was cultured, and the test groups were respectively containing 2, 5 or 10 U / ml of heparin.
[0062] As shown in Table 3, the group cultured with 2U / ml heparin had the most stable cell expansion trend, and the total number of cells obtained on the 20th day of culture was 1.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com