Method for large-scale production of recombinant human butyrylcholinesterase using transgenic animal mammary gland biological platform
A butyrylcholinesterase and transgenic technology, applied in the field of producing recombinant human butyrylcholinesterase, can solve the problems of low transgenic efficiency, unfavorable quality control and subsequent processing, no in vivo enzymatic activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] Example 1. Construction and preparation of transgenic expression cassettes
[0199] Experimental steps:
[0200] (i) Plasmid pUC19 (Promega) was cut with SacI and BamHI endonucleases, and ligated to an insulator fragment from the upstream region of the chicken β-globin gene (Proc Natl Acad Sci USA94:575-80, 1997). The insulator fragment is made using cis primers containing SacI, SacII, NotI, SalI sites and part of the chicken β-globin gene upstream region DNA sequence such as SEQ ID NO.: 7 (5'GAC ACT GAG CTC CAC CGC GGA CTG CGG CCG CTC GTC GAC GGG ACAGCC CCC CCC CAA AGC CCC CAG3'), and the reverse primer containing BamHI, XhoI site and part of the chicken β-globin gene upstream region DNA sequence such as SEQ ID NO.: 8 (5'AGC GTC GGA TCC ACT TGC GTC CTC GAG GCC CCATCC TCA CTG ACT CCG TCC TGG AGT TG3'), amplified from chicken genomic DNA by PCR. The PCR product was divided into two parts, cut with SacI-XhoI or SalI-BamHI respectively, and then joined together through S...
Embodiment 2
[0205] Example 2. Production of transgenic sheep
[0206] Embryonic cells were isolated from sheep embryos on day 27-30, mainly containing embryonic fibroblasts, and cultured in Dulbecco's modified Eagle medium at 38°C, 5% carbon dioxide, supplemented with 20% fetal bovine serum (FBS) and 20 μg / ml gentamicin. When the cells reached 70% confluence, they were treated with 0.05% trypsin-EDTA, collected, counted, and frozen in aliquots of 10% DMSO and 90% FBS. A small number of cells were placed on slides for cytogenetic analysis. Chromosomally normal frozen cells were thawed for transfection of transgene expression construct DNA to generate stable cell lines. Stable cell lines were generated by lipid-mediated gene transfer.
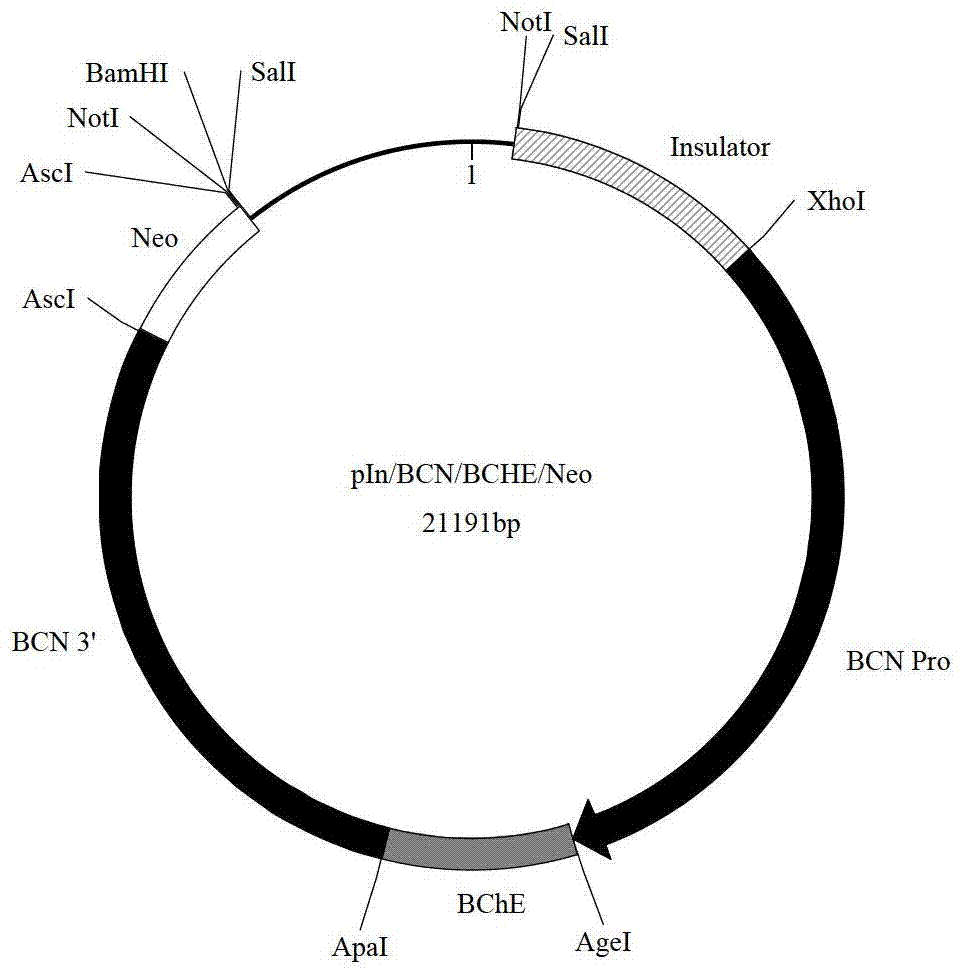
[0207] The above-mentioned transgene expression cassette (i.e. pIn / BCN / BCHE / Neo plasmid) DNA fragment of embodiment 1, comprises insulator, goat β-casein promoter, human butyrylcholinesterase cDNA sequence, β-casein gene 3' end and Neo fragments, cleaved...
Embodiment 3
[0211] Example 3. Detection and breeding of transgenic breeding sheep and their offspring
[0212] About 4 days after the birth of the cloned new sheep, blood was taken for PCR analysis to confirm the transgene. There are three sets of PCR reaction primers.
[0213] The first set of primers such as SEQ ID NO.:15 and 16 (5'CTT CCG TGG CCA GAA TGG AT3' / 5'CAT CAGAAG TTA AAC AGC ACA GTT AGT3') from the 3' end of human butyrylcholinesterase cDNA to phase A 510bp DNA fragment was amplified from the β-casein gene exon 7 fragment adjacent to the BCN-BCHE fragment.
[0214] The second set of primers such as SEQ ID NO.: 17 and 18 (5'AGG AGC ACA GTG CTC ATC CAG ATC3' / 5'GAC GCC CCA TCC TCA CTG ACT3') amplifies a 910bp DNA fragment from the insulator fragment.
[0215] The third set of primers such as SEQ ID NO.: 19 and 20 (5'GAG GAA CAA CAG CAA ACA GAG3' / 5'ACCCTA CTG TCT TTC ATC AGC3') amplifies the goat endogenous β-casein gene 360bp fragment to Make sure that the extracted DNA is fre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com