HER-2 humanized monoclonal antibody long-acting sustained-release preparation and preparation method thereof
A HER-2 and monoclonal antibody technology, applied in the field of medicine, can solve the problems of low drug utilization rate, poor patient compliance, tumor recurrence in situ, etc., and achieve the effect of reducing tumor recurrence rate and drug toxicity and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
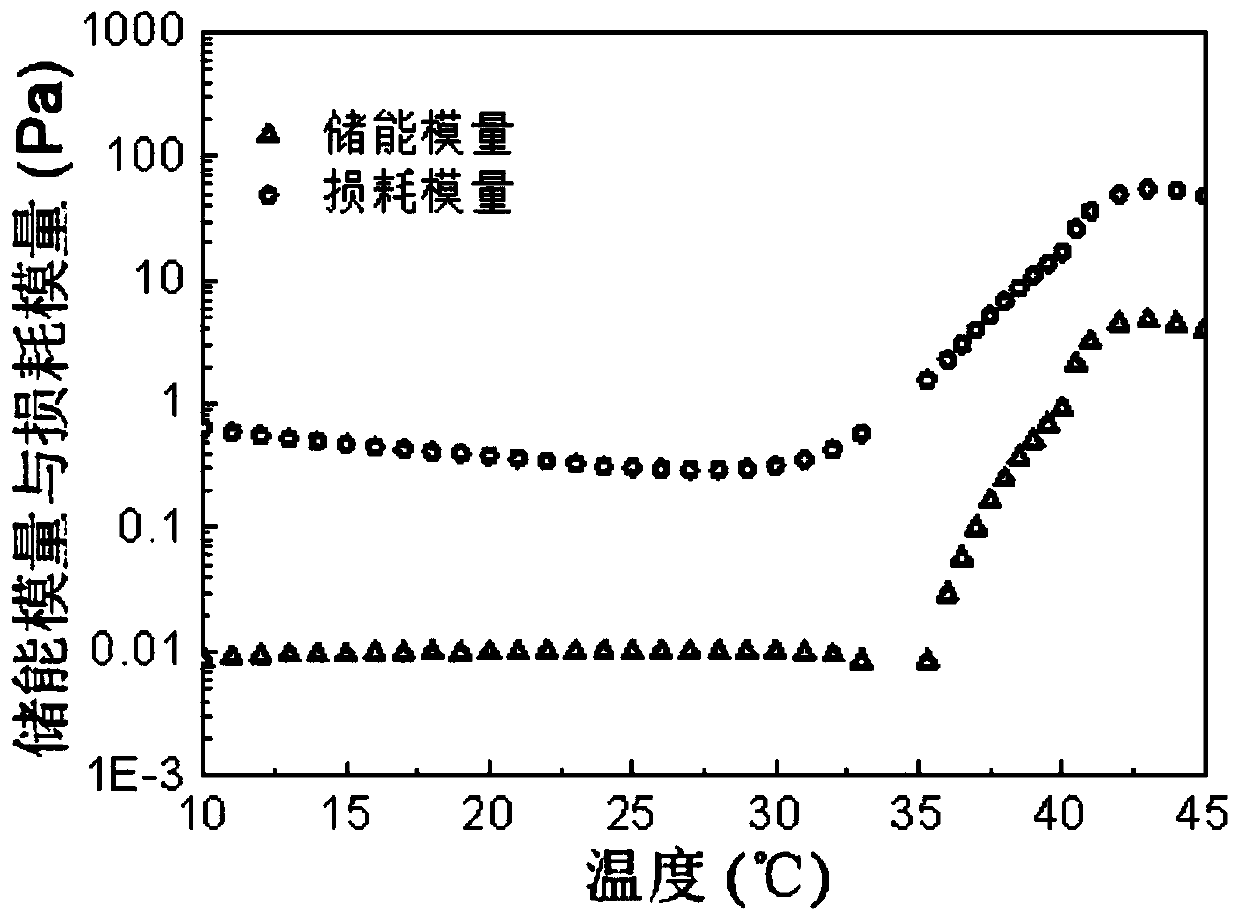
[0054] An amphiphilic block copolymer. Weigh 30g of PEG 1500 in a 250mL three-neck bottle, seal each port with vacuum ester, and remove water under vacuum at 130°C for 3h under mechanical stirring. Then cool down to 100°C with argon, add 40g of D,L-lactide and 8g of glycolide, stir well; add toluene solution containing 60mg of stannous octoate, vacuumize for 15min to remove toluene, then heat up to 150°C under argon The reaction was carried out under gas protection for 12h. Then lower the temperature to 110°C and vacuumize for 3 hours to remove unreacted monomers, then wash the initial product three times with 80°C deionized water, and freeze-dry to obtain the final product, obtaining the BAB type triblock polymer PLGA-PEG-PLGA, The yield is about 80%. The properties of the block polymer were measured, and the results are shown in Table 1. The number-average and weight-average molecular weight (M n ,M w ) are 5550 and 6330 respectively, molecular weight distribution coeffi...
Embodiment 2
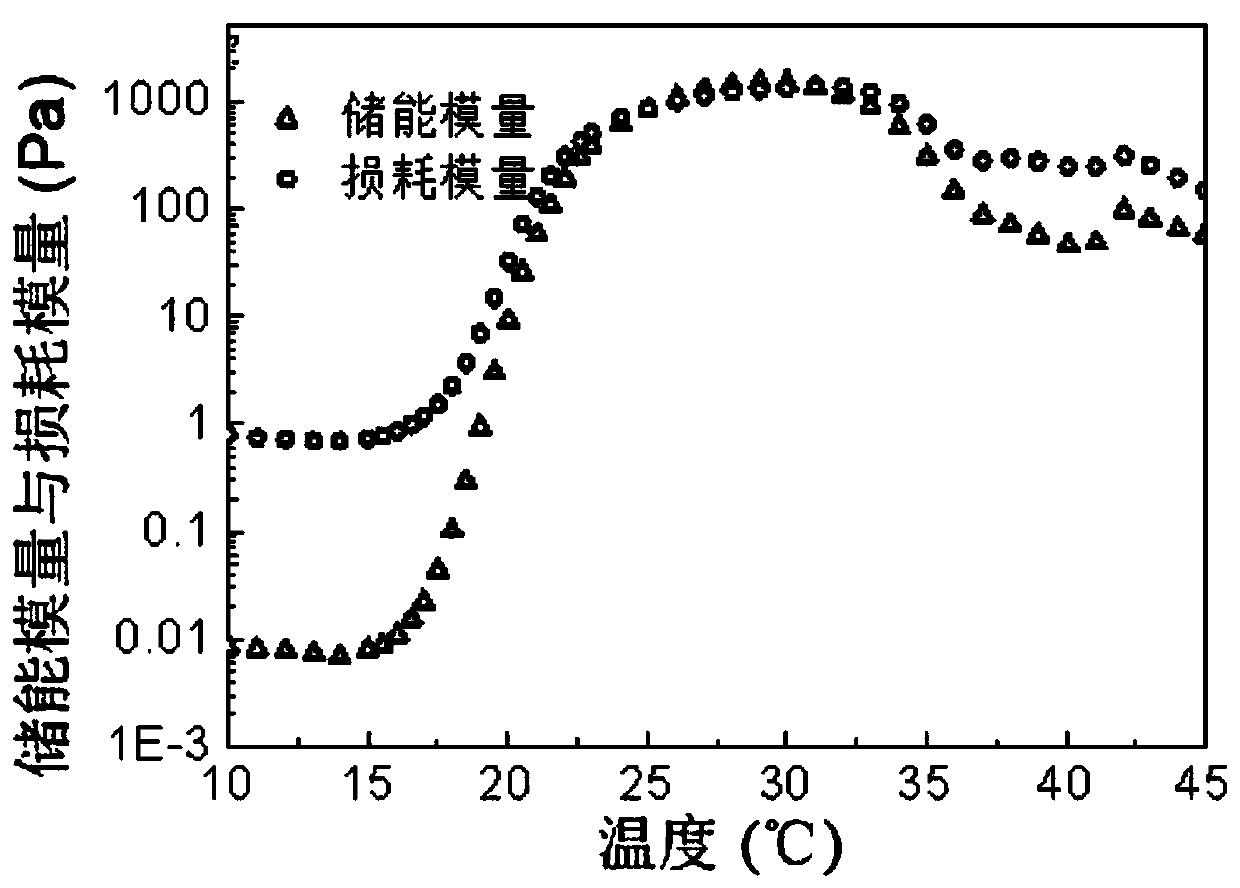
[0056] An amphiphilic block copolymer. Weigh 30g of PEG 1500 in a 250mL three-neck bottle, seal each port with vacuum ester, and remove water under vacuum at 130°C for 3h under mechanical stirring. Then cool down to 100°C with argon, add a total of 48g of D-lactide and glycolide with a molar ratio of 1:1, and stir thoroughly; add toluene solution containing 60mg of stannous octoate, vacuumize for 15min to remove toluene and then raise the temperature React at 150°C for 12h under the protection of argon. Then lower the temperature to 110°C and vacuumize for 3 hours to remove unreacted monomers, then wash the initial product three times with 80°C deionized water, and freeze-dry to obtain the final product to obtain the BAB type triblock polymer PDLGA-PEG-PDLGA, The yield is about 87%. The properties of the block polymer were measured, and the results are shown in Table 1. The number-average and weight-average molecular weight (M n ,M w ) are 5100 and 6320 respectively, molec...
Embodiment 3
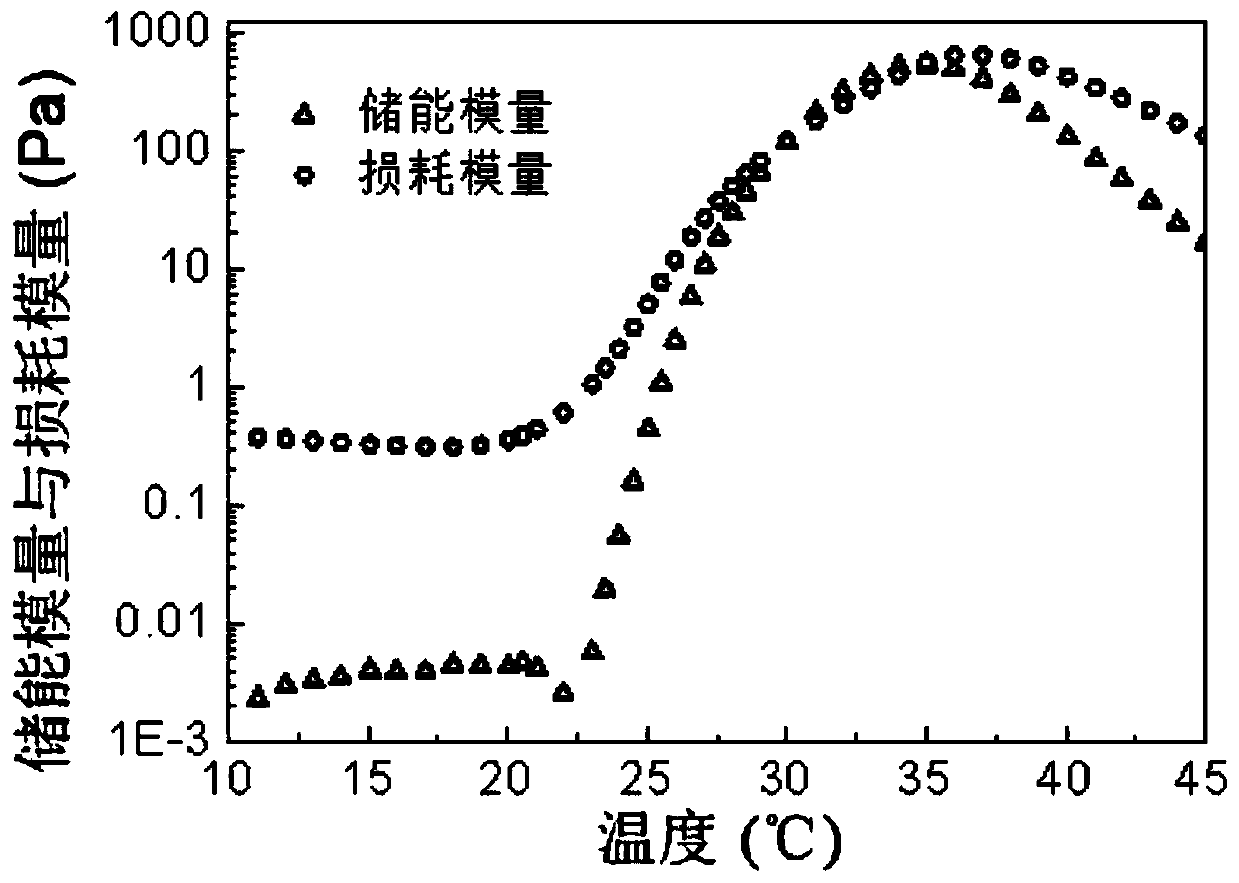
[0058] An amphiphilic block copolymer. Weigh 20g of PEG 1000 in a 250mL three-neck bottle, seal each port with vacuum ester, and remove water under vacuum at 130°C for 3h under mechanical stirring. Then cool down to 100°C with argon gas, add 46.6g of D,L-lactide and 9.4g of glycolide, stir well; add toluene solution containing 60mg of stannous octoate, vacuumize for 15min to remove toluene, then heat up to 150°C The reaction was carried out under the protection of argon for 12h. Then lower the temperature to 110°C and vacuumize for 3 hours to remove unreacted monomers, then wash the initial product three times with 80°C deionized water, and freeze-dry to obtain the final product, obtaining the BAB type triblock polymer PLGA-PEG-PLGA, The yield is about 80%. The properties of the block polymer were measured, and the results are shown in Table 2. The number-average and weight-average molecular weight (M n ,M w ) are 5590 and 7040 respectively, molecular weight distribution c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com