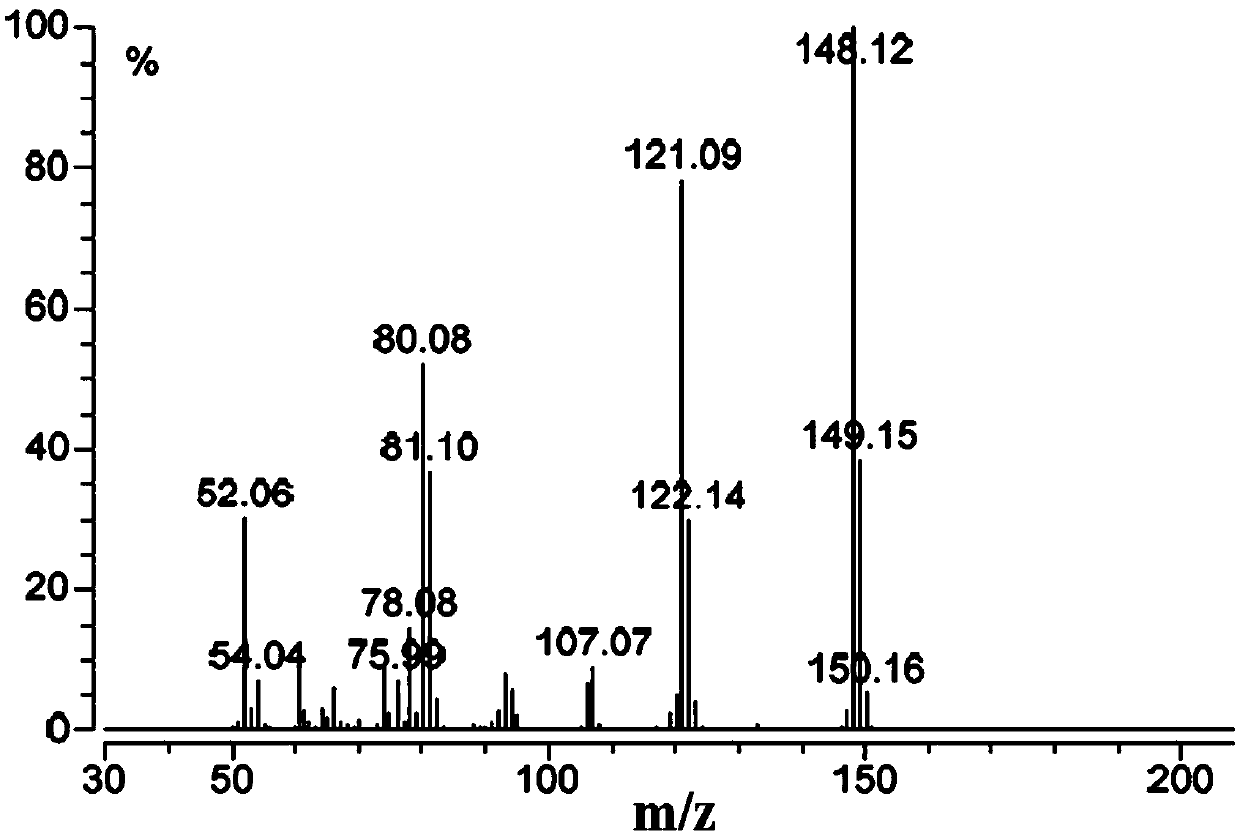
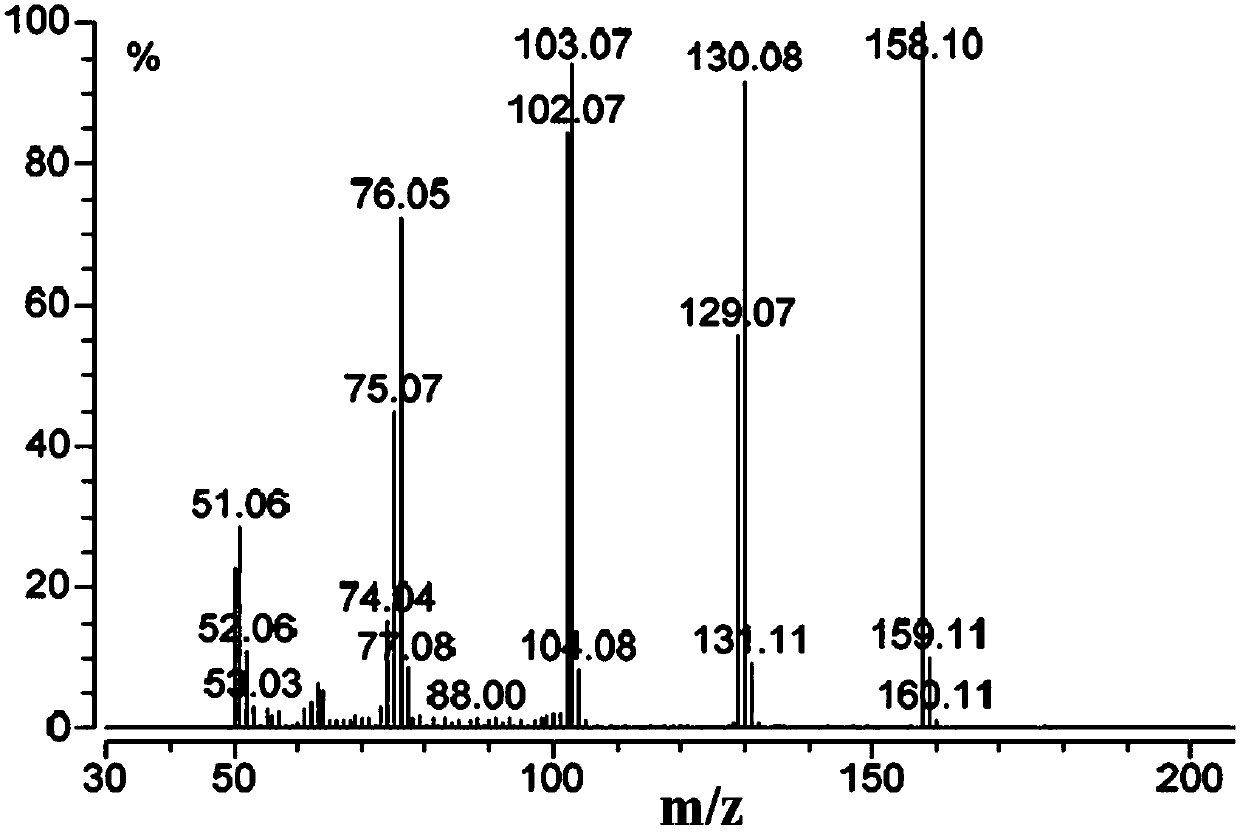
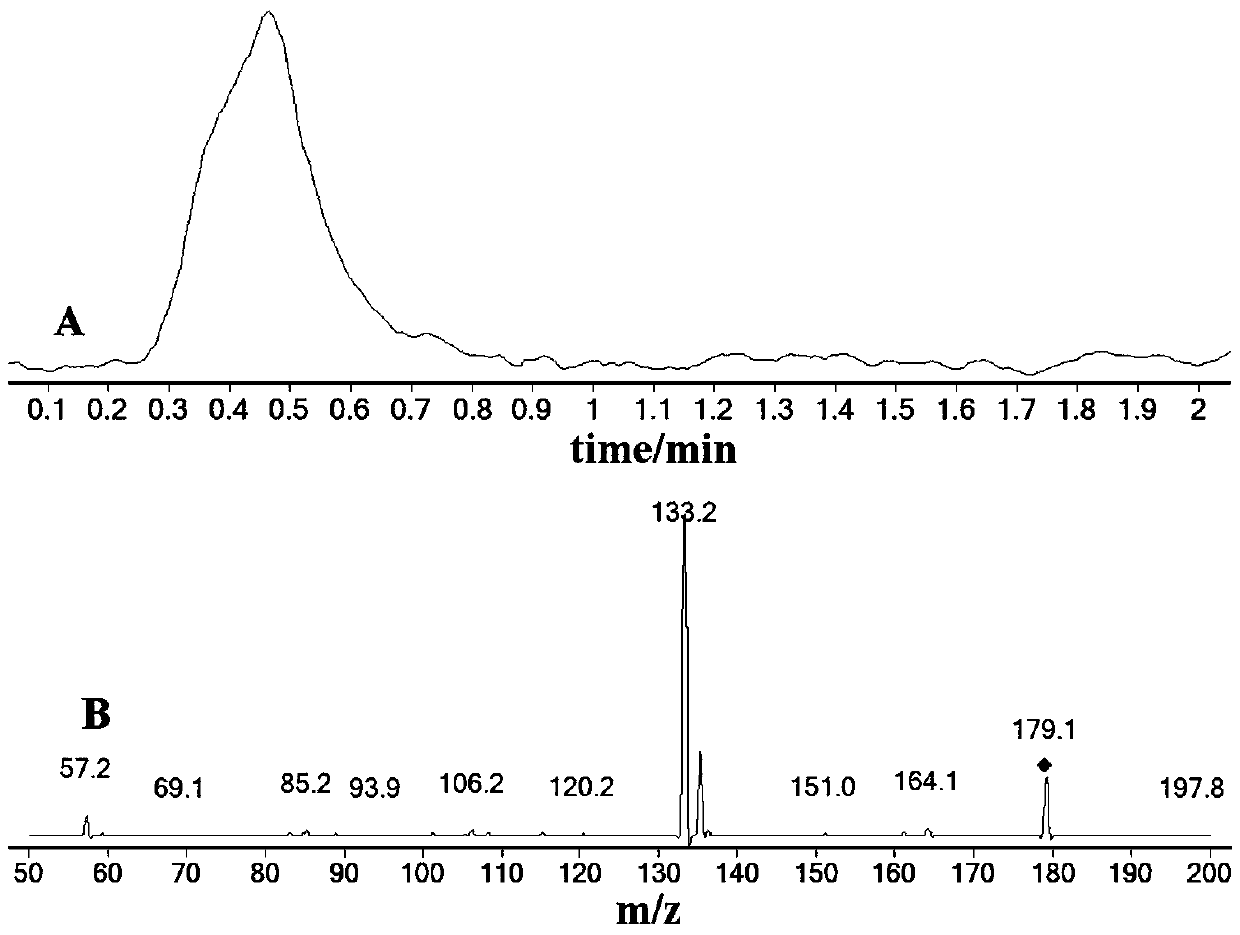
Synthesis method of stable isotope labeled quinoxaline-2-carboxylic acid
A stable isotope and synthetic method technology, applied in the field of organic compound synthesis, can solve the problem of no synthetic preparation method found, and achieve the effect of fewer experimental steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] A kind of synthetic method of the quinoxaline-2-carboxylic acid of stable isotope label provided by the invention comprises the following steps:
[0020] S1: After condensation reaction of 40% aqueous solution of methylglyoxal with stable isotope-labeled aniline, 2-methylquinoxaline labeled with stable isotope is reacted; The molar ratio is 1.5~1.2:1, and the reaction solvent is methanol, ethanol or tetrahydrofuran;
[0021] S2: Next, selenium dioxide is used to oxidize stable isotope-labeled 2-methyl-quinoxaline to form stable isotope-labeled 2-formaldehydequinoxaline; selenium dioxide and stable isotope-labeled 2-methylquinoxaline The molar ratio is 1.5~2.0:1, and the reaction solvent is 1,4-dioxane, ethyl acetate or tetrahydrofuran;
[0022] S3: Finally, the stable isotope-labeled 2-formaldehydequinoxaline is reacted with an oxidizing agent to obtain stable isotope-labeled quinoxaline-2-carboxylic acid; The ratio is 1.5-2.0:1, the oxidizing agent is hydrogen peroxi...
Embodiment 1
[0028] S1) After dissolving 1.12g of stable isotope-labeled o-phenylenediamine in 20mL of methanol, 2.7g of 40% aqueous solution of aceglyoxal was added dropwise, and reacted at 40°C for 24 hours. After cooling to room temperature, 80 mL of ethyl acetate was added, followed by washing with 1M hydrochloric acid (30 mL), water (30 mL) and saturated brine. After drying over anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and separate and purify by column chromatography. The eluent was selected to elute the product with n-hexane:ethyl acetate=6:1. Finally, 1.19 g of stable isotope-labeled 2-methylquinoxaline was obtained as an orange oily liquid with a yield of 80.1%.
[0029] S2) Dissolve 1.19 g of the stable isotope-labeled 2-methylquinoxaline obtained above in 50 mL of 1,4-dioxane, then add 1.35 g of selenium dioxide, and react at 100 degrees for 4 hours. After the reaction, cool down, remove the solid by suction filtration, remove part of the sol...
Embodiment 2
[0032] S1) After dissolving 1.68g of stable isotope-labeled o-phenylenediamine in 20mL of ethanol, 3.24g of 40% aqueous solution of methylglyoxal was added dropwise, and reacted at 60°C. React for 12 hours. After the end, the aftertreatment was the same as step (1) in Example 1 to obtain 1.84 g of stable isotope-labeled 2-methylquinoxaline, with a yield of 83.3%.
[0033] S2) Dissolve the stable isotope-labeled 2-methylquinoxaline obtained above in 60 mL of ethyl acetate, then add 2.68 g of selenium dioxide, and react at 80 degrees for 4.5 hours. After the reaction was completed, it was cooled to room temperature, and the aftertreatment was the same as step S2) in Example 1 to obtain 1.63 g of the target product with a yield of 81.1%.
[0034] S3) The stable isotope-labeled 2-formaldehydequinoxaline obtained above was dissolved in 30 mL of N,N-dimethylformamide, and 6.14 g of potassium hydrogen persulfate was added to react at room temperature for 2 hours. After the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com