Preparation method of parecoxib meta-isomer impurity
A parecoxib and isomerization technology, applied in the field of medicinal chemical synthesis, can solve the problems of uncontrollable safety factors, cumbersome steps, high cost, etc., and achieve good industrial application prospects, stable process, and reduce by-products. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
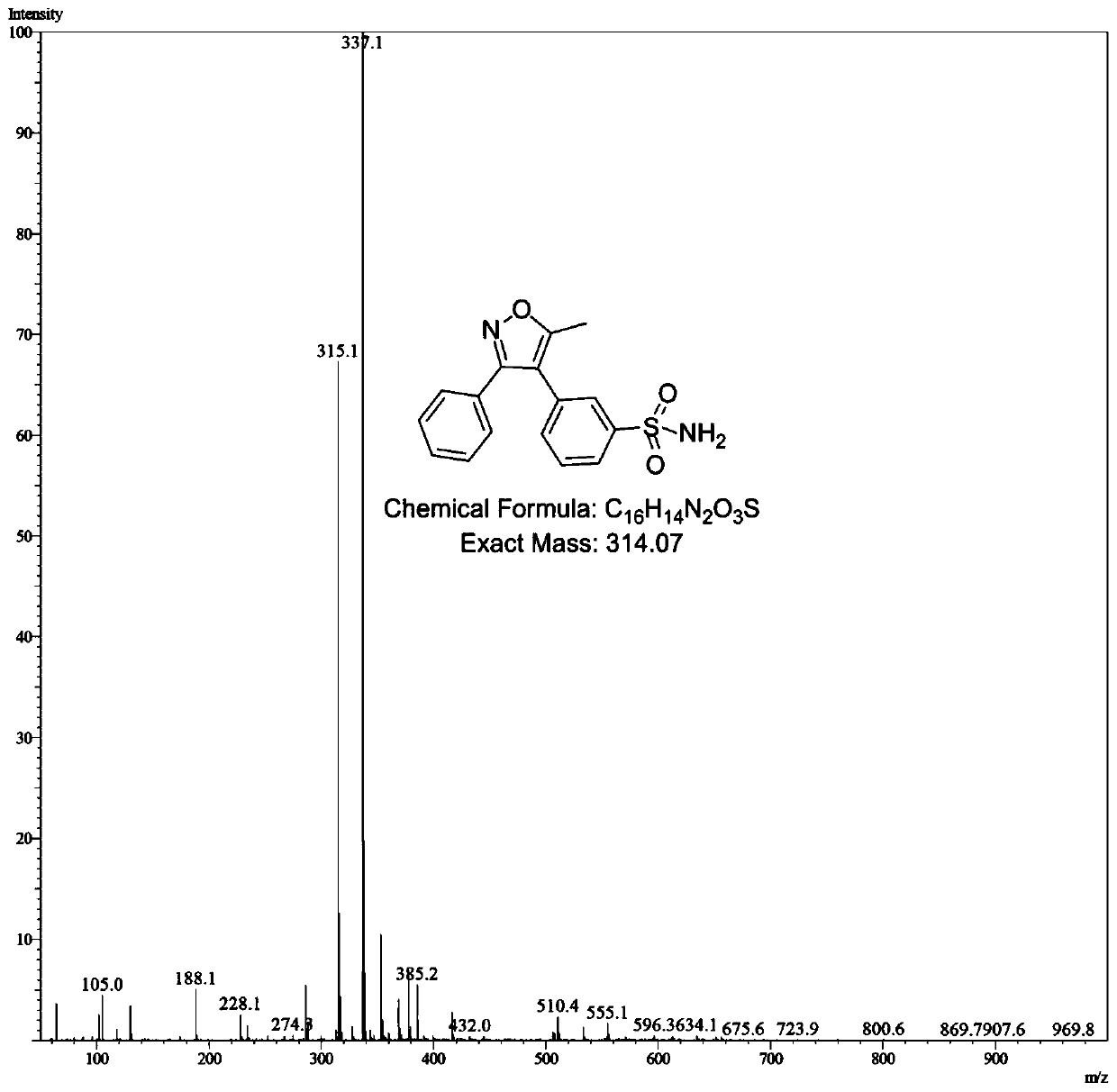
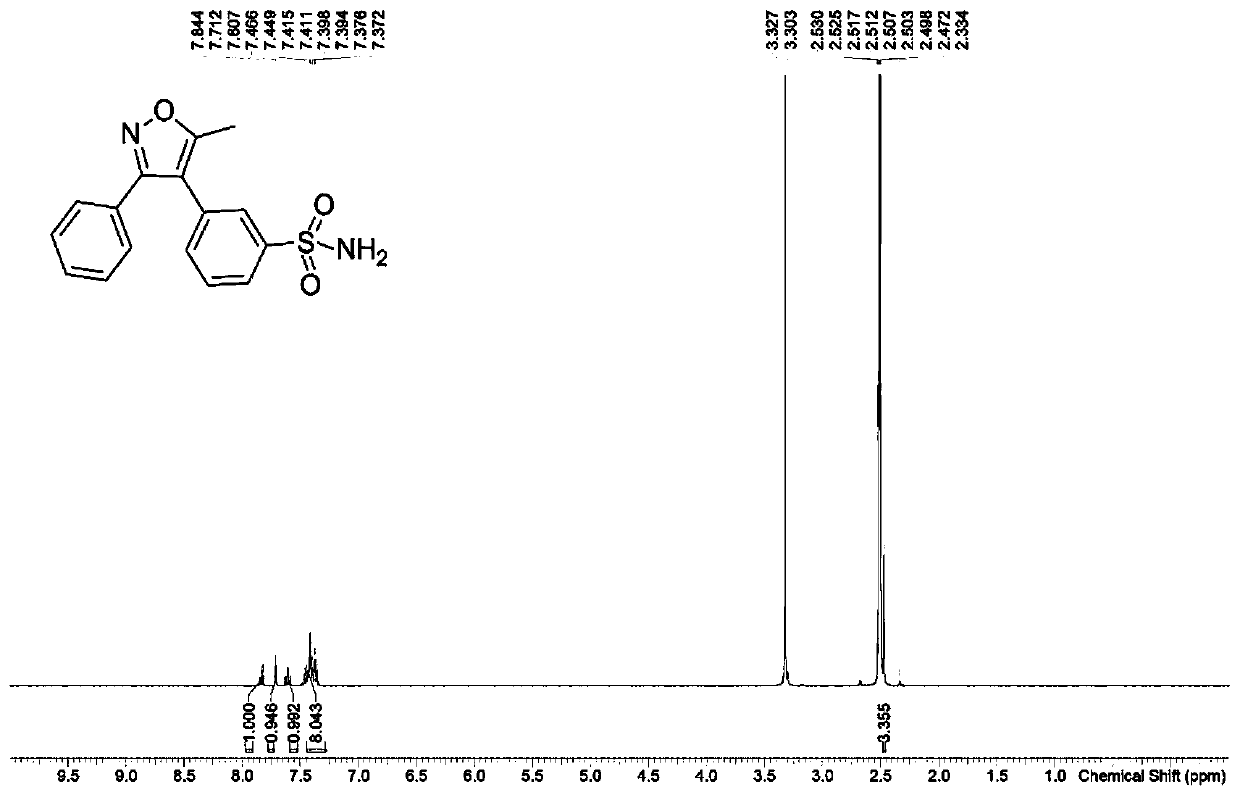
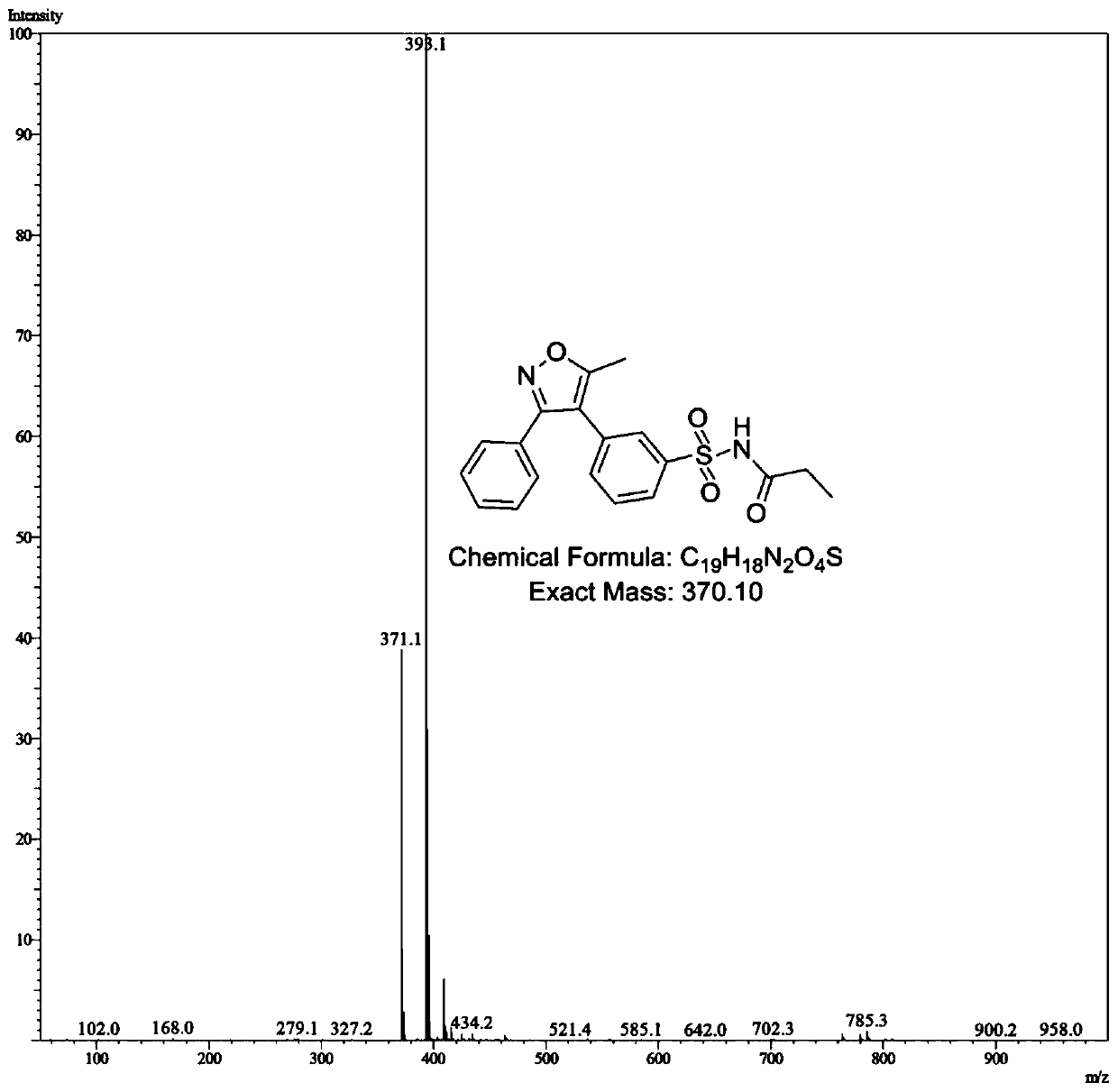
[0038] The preparation method of the parecoxib meta-isomeric impurity N-[[3-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]propionamide in this embodiment is as follows:
[0039] Step S1, the preparation of N-[3-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonamide:
[0040] Add 5-methyl-3-phenylisoxazole-4-carboxylic acid (2.03g, 10mmol, 1.0eq), 3-bromobenzenesulfonamide (2.36g, 10mmol, 1.0eq), silver carbonate to a 100mL three-necked flask (6.89g, 25mmol, 2.5eq), triphenylphosphine (1.57g, 6mmol, 0.6eq), bis(acetylacetonate)palladium(II) (609mg, 2mmol, 0.2eq) and anhydrous N-methylpyrrolidone ( 16mL), then replaced with nitrogen for 3 times, heated to 160°C for 24 hours, cooled to room temperature, added water (30mL) and ethyl acetate (50mL), stirred for 10 minutes, filtered with diatomaceous earth, separated, and the water phase Then extract 2 times with ethyl acetate (30mL), combine the organic phases, wash with saturated brine and water successively, then dry with anhydrou...
Embodiment 2
[0059] The preparation method of the parecoxib meta-isomeric impurity N-[[3-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]propanamide of the present embodiment is mainly the same as The same as in Example 1, the difference is that the method is as follows:
[0060] Step S1, the preparation of N-[3-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonamide:
[0061] Add 5-methyl-3-phenylisoxazole-4-carboxylic acid (2.03g, 10mmol, 1.0eq), 3-bromobenzenesulfonamide (2.36g, 10mmol, 1.0eq), silver carbonate to a 100mL three-necked flask (5.52g, 20mmol, 2.0eq), triphenylphosphine (1.31g, 5mmol, 0.5eq), palladium chloride (89mg, 0.5mmol, 0.05eq) and anhydrous N,N-dimethylpropenyl urea ( 10mL), then replaced with nitrogen for 3 times, heated to 130°C for 24 hours, cooled to room temperature, added water (30mL) and ethyl acetate (50mL), stirred for 10 minutes, filtered with diatomaceous earth, separated, and the aqueous phase Then extract 2 times with ethyl acetate (30mL), combine the orga...
Embodiment 3
[0066] The preparation method of the parecoxib meta-isomeric impurity N-[[3-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]propanamide of the present embodiment is mainly the same as The same as in Example 1, the difference is that the method is as follows:
[0067] Step S1, the preparation of N-[3-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonamide:
[0068] Add 5-methyl-3-phenylisoxazole-4-carboxylic acid (2.03g, 10mmol, 1.0eq), 3-bromobenzenesulfonamide (2.36g, 10mmol, 1.0eq), silver carbonate to a 100mL three-necked flask (11.03g, 40mmol, 4.0eq), triphenylphosphine (2.10g, 8mmol, 0.8eq), palladium trifluoroacetate (665mg, 2mmol, 0.2eq) and anhydrous N,N-dimethylacetamide (20mL ), then replaced with nitrogen for 3 times, raised the temperature to 160°C for 24 hours, cooled to room temperature, added water (30mL) and ethyl acetate (50mL), stirred for 10 minutes, filtered with diatomaceous earth, separated, and the water phase was re- Extracted twice with ethyl acetate (30m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com