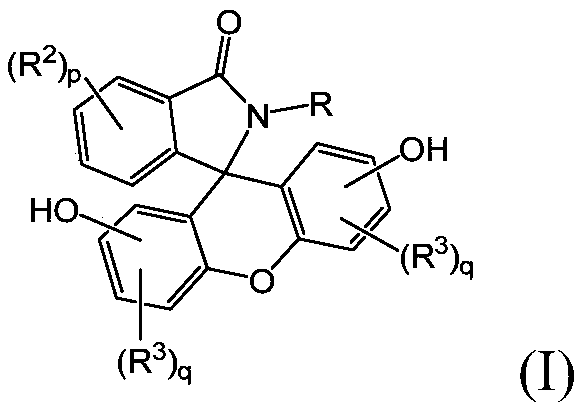
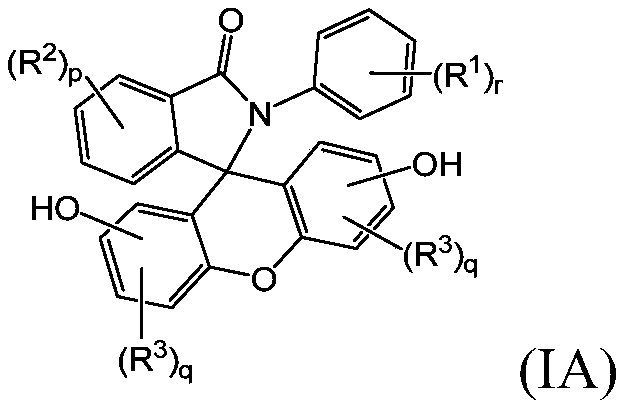
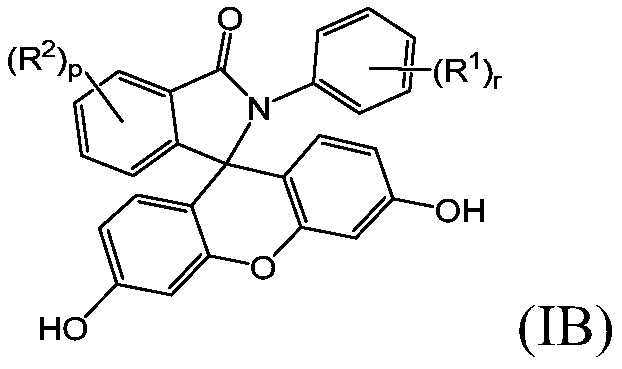
2-hydrocarbyl-3-(dihydroxyfluoresceinyl)phthalimidine monomers, methods of manufacture, and copolymers derived therefrom
A phthalimide and fluorescein technology, applied in organic chemistry and other fields, can solve the problems of time-consuming, resource-intensive, and difficult purification of phenolphthalein derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093]140 g of aniline and 40 ml of 33% HCl (33 wt% in water) were combined in a four necked round bottom flask equipped with an overhead condenser, nitrogen inlet and overhead stirrer. The reaction mixture was stirred for 1 hour to provide the aniline hydrochloride. Then 100 g of fluorescein was added to the reaction mixture, and the resulting mixture was heated at 170° C. for 30 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) in a 1:1 solution of ethyl acetate and hexanes using silica gel plates. After the reaction was completed, the temperature was lowered to 120° C. and 150 ml of HCl (33 wt % in water) was added thereto to convert the remaining aniline into the corresponding hydrochloride. Then 400 ml of deionized water were added and the resulting mixture was stirred for 1 hour. The solid was collected by filtration and dried at 120°C to provide the crude phthalimide product. The crude product was 81.7% pure as determined by HPLC. ...
Embodiment 2
[0095] 50 g of the crude phthalimide product from Example 1 and 500 ml of aqueous NaOH (10 wt% in water) were combined in a three necked round bottom flask equipped with nitrogen inlet and overhead stirrer. The reaction mixture was stirred for 1 hour, then filtered to remove insoluble components. The filtrate was collected and combined with 10 wt% activated carbon (based on the weight of the crude product), stirred at 25°C for 2 hours, and then filtered on celite. The charcoal treatment was repeated a second time and the resulting filtrate was precipitated by addition of HCl (diluted). The resulting solid was isolated by filtration and washed with deionized water to remove residual aniline chloride. The semi-purified phthalimide product was 99.3% pure as determined by HPLC.
Embodiment 3
[0097] The semi-purified phthalimide product was combined with a mixture of methanol and water (90:10 vol / vol) and dissolved by heating at 60°C to form a solution (20 wt% product). Activated carbon (10 wt% based on the weight of the crude product) was added to the solution, and the resulting mixture was stirred at 60°C for 1 hour. The mixture was filtered to separate the activated carbon, then diluted with deionized water (10 parts by volume based on the total volume of the methanol and water mixture). The mixture was stirred at 25°C for 30 minutes. The purified phthalimide product was then isolated by filtration. The purified phthalimide product was 99.8% pure as determined by HPLC.
[0098] by liquid chromatography-mass spectrometry (LC-MS) and proton nuclear magnetic resonance ( 1 H-NMR) spectrum further characterizes the purified phthalimide product. LC-MS: m / z = 406 Daltons [M-H]. 1 H NMR: (DMSO-d 6 )=9.90ppm(s,2H), 7.29ppm(d,1H), 7.60ppm(m,2H), 7.15ppm(m,4H), 6.64p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com