Organic compound and organic electroluminescent element comprising same
A compound and chemical formula technology, applied in the field of organic electroluminescent elements, can solve the problems of unsatisfactory service life, poor thermal stability, and low glass transition temperature of light-emitting elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
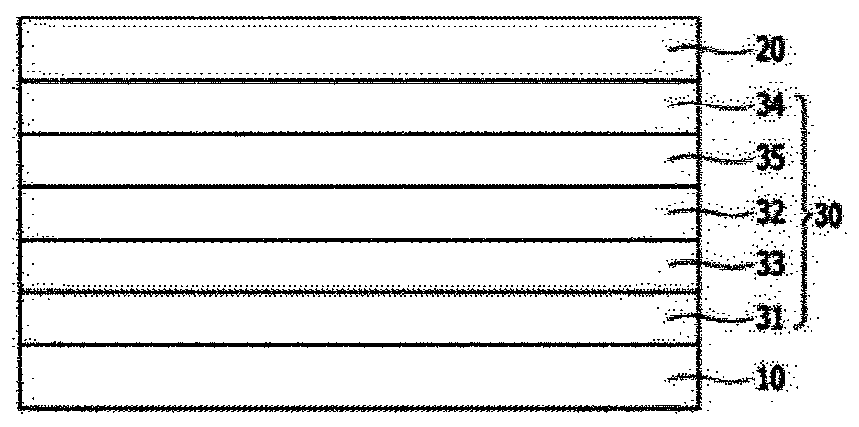
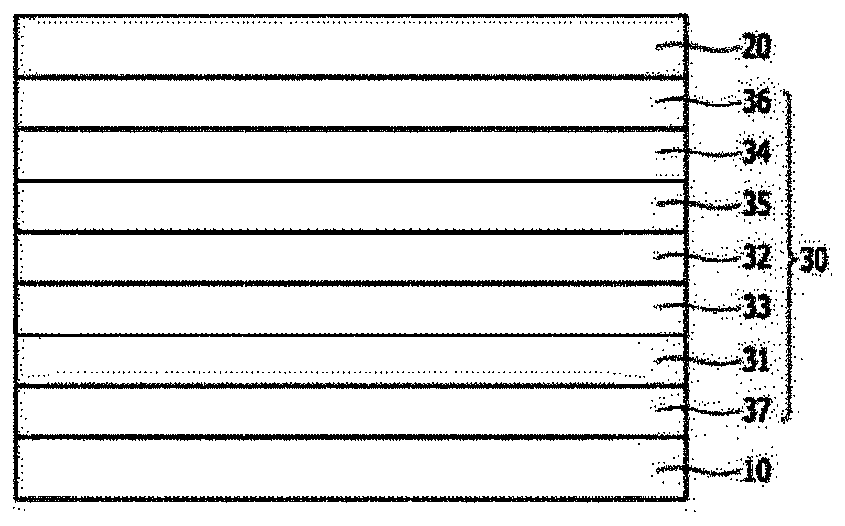
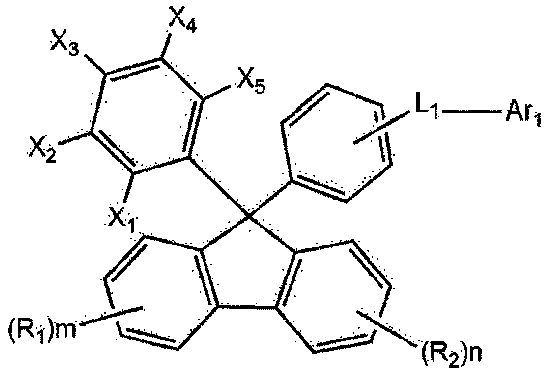
Image
Examples
Embodiment
[0206] [Preparation example 1] Synthesis of core (Core) 1
[0207] (4-chlorophenyl)(2-phenylbenzo[d] Azol-6-yl)methanone ((4-chlorophenyl)(2- Synthesis of phenylbenzo[d]oxazol-6-yl)methanone)
[0208]
[0209]Add 6-bromo-2-phenylbenzo[d] to the reactor oxazole (6-bromo-2-phenylbenzo[d]oxazole) (100g, 364.8mmol) was poured into 1000ml of THF, stirred, added to a dry ice bath, and the internal temperature was set to -78°C. After slowly injecting 2.5M n-BuLi (133.7 ml, 334.4 mmol) with a syringe, the mixture was stirred for 30 minutes. 4-Chlorobenzaldehyde (4-chlorobenzaldehyde) (42.7g, 304mmol) was dissolved in 100ml of THF and slowly added dropwise. Slowly warm up to room temperature. After concentrating the reaction, add I 2 (203.7g, 802.6mmol), K 2 CO 3 (166.4g, 1203.8mmol), t-BuOH800ml, heated to reflux for 7 hours. After the reaction, extract with ethyl acetate, then add MgSO 4 filter.
[0210] After the reaction, extract with dichloromethane, add Mg...
Synthetic example 1
[0254] [Synthesis Example 1] Synthesis of Compound Inv 1
[0255]
[0256] The core 1 (5g, 8.9mmol) of [Preparation Example 1] and 2-chloro-4,6-diphenyl-1,3,5-triazine (2-chloro-4,6-diphenyl-1,3 ,5-triazine) (2.86g, 8.9mmol) and Pd (PPh 3 ) 4 (0.3g, 0.26mmol), K 2 CO 3 (3.7g, 26.7mmol) was added to toluene 80ml, EtOH 20ml, H 2 O 20ml, heated to reflux for 12 hours. After the reaction, extract with dichloromethane, add MgSO 4 filter. After the solvent of the filtered organic layer was removed, the target compound Inv 1 (4.3 g, yield 72%) was obtained by column chromatography.
[0257] [LCMS]: 666.7
Synthetic example 2
[0258] [Synthesis Example 2] Synthesis of Compound Inv 12
[0259]
[0260] Using 2-chloro-4,6-bis(dibenzo[b,d]furan-3-yl)-1,3,5-triazine (2-chloro-4,6-bis(dibenzo[b,d] ]furan-3-yl)-1,3,5-triazine) (4.7g, 10.68mmol) instead of 2-chloro-4,6-diphenyl-1,3,5-triazine, in addition, implement The target compound Inv 12 (5.6 g, yield 74%) was obtained by the same procedure as [Synthesis Example 1].
[0261] [LCMS]: 846.9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com