Water-based compound adjuvant, application thereof and vaccine for cats
An adjuvant and water-based technology, applied in the field of biomedicine, can solve the problems of easy allergy and high irritation at the injection site, and achieve the effect of less allergy and less irritation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
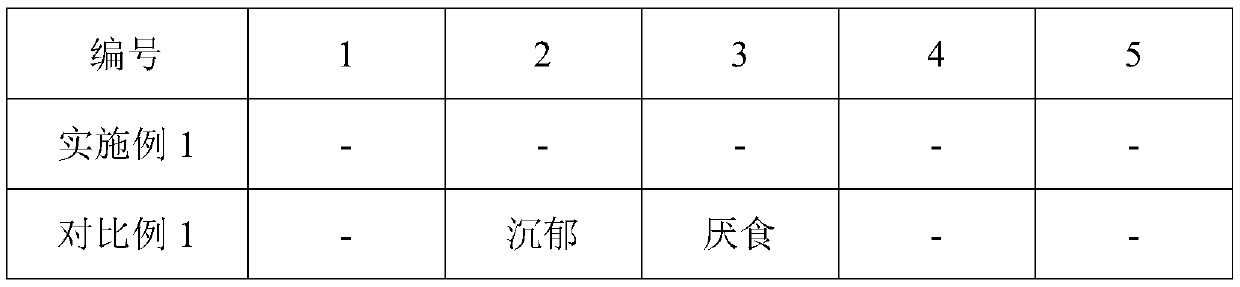
Embodiment 1
[0025] This embodiment provides an aqueous composite adjuvant and inactivated cat distemper vaccine, wherein the preparation method of the aqueous composite adjuvant is as follows: first weigh 0.5 mg of mycobacterium vaccae peptide for injection, 5 mg of astragalus polysaccharide, interleukin-7 10 μg, 0.1 mL of aluminum hydroxide gel, 100 mg of stabilizer, and set aside; then, under normal temperature conditions, mix the above-mentioned Mycobacterium vaccae peptides for injection, astragalus polysaccharide, interleukin-7, aluminum hydroxide gel, stabilizer and Sterile deionized water was mixed to prepare 1 mL of aqueous composite adjuvant. Wherein, Mycobacterium vaccae peptide for injection is a commercially available product of General Biosystems (Anhui) Co., Ltd.; Astragalus polysaccharide is a commercially available product of Xi'an Wanfang Biotechnology Co., Ltd., which is a water-soluble powder of 90% purity; interleukin- 7 is feline interleukin-7 expressed by Shanghai Bi...
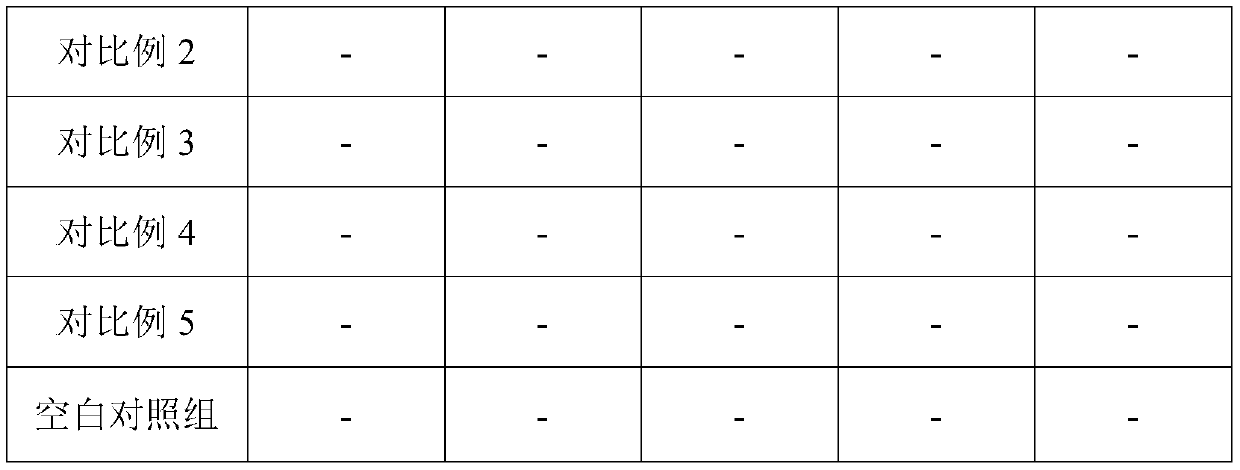
Embodiment 2
[0028] This embodiment provides a water-based composite adjuvant and an inactivated vaccine for feline infectious rhinotracheitis, wherein the preparation method of the water-based composite adjuvant is as follows: first weigh 3 mg of mycobacitracin for injection, 1 mg of astragalus polysaccharide, Interleukin-7 100μg, aluminum hydroxide gel 0.2mL, stabilizer 300mg, set aside; then, under normal temperature conditions, the above weighed mycobacterium vaccae peptides for injection, astragalus polysaccharide, interleukin-7, aluminum hydroxide gel, stabilizer The adjuvant was mixed with sterile deionized water to prepare 1 mL of aqueous composite adjuvant. Wherein, Mycobacterium vaccae peptide for injection is a commercially available product of General Biosystems (Anhui) Co., Ltd.; Astragalus polysaccharide is a commercially available product of Xi'an Wanfang Biotechnology Co., Ltd., which is a water-soluble powder of 90% purity; interleukin- 7 is feline interleukin-7 expressed ...
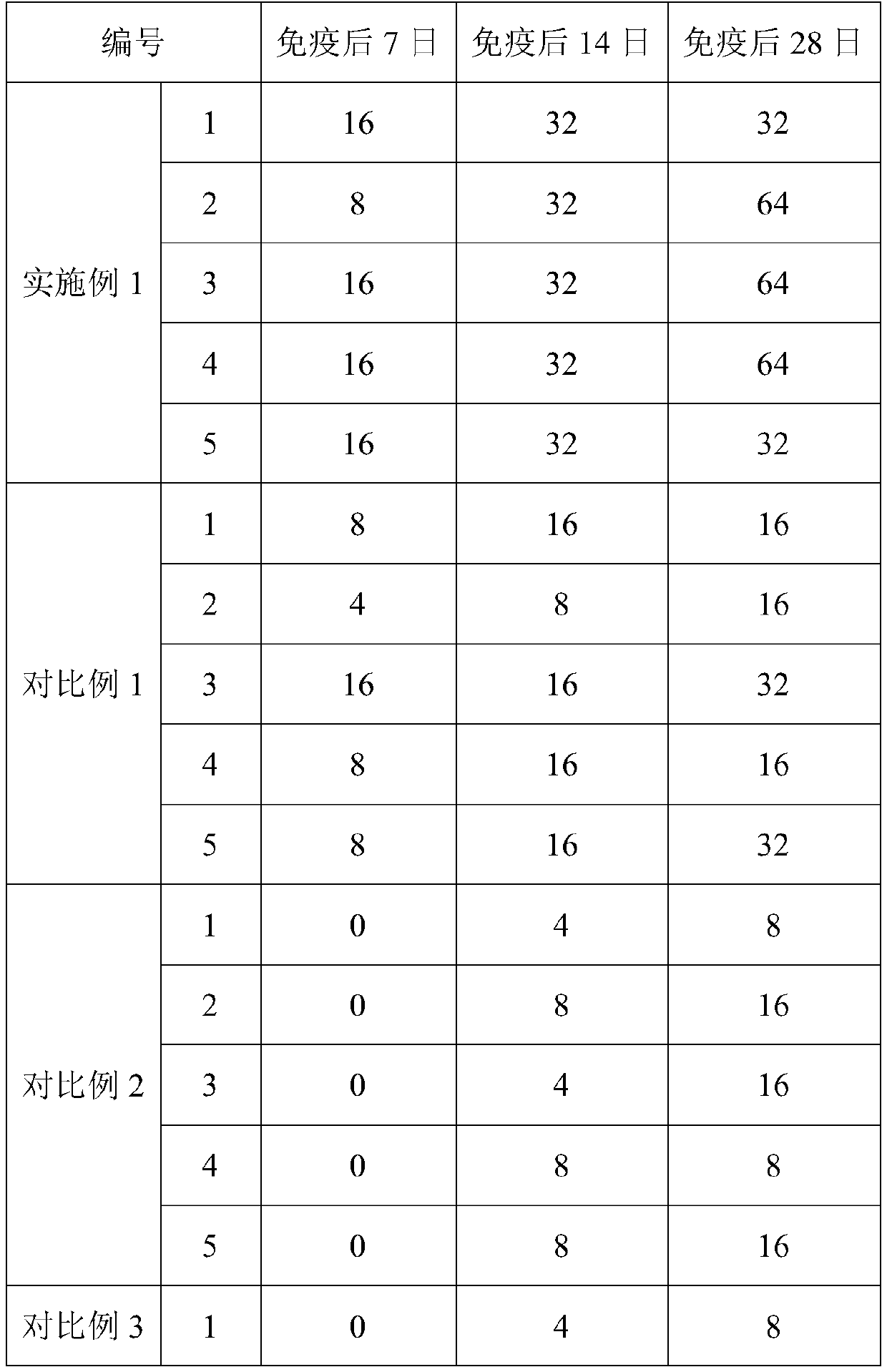
Embodiment 3
[0031] This embodiment provides an aqueous composite adjuvant and inactivated feline calicivirus vaccine, wherein the preparation method of the aqueous composite adjuvant is as follows: first weigh 2 mg of mycobacterium vaccae peptide for injection, 3 mg of astragalus polysaccharide, interleukin- 7 50 μg, 0.15 mL of aluminum hydroxide gel, 300 mg of stabilizer, for later use; then, under normal temperature conditions, mix the above-weighed mycobacterium vaccae peptides for injection, astragalus polysaccharide, interleukin-7, aluminum hydroxide gel, stabilizer Mix with sterile deionized water to prepare 1 mL of aqueous composite adjuvant. Wherein, Mycobacterium vaccae peptide for injection is a commercially available product of General Biosystems (Anhui) Co., Ltd.; Astragalus polysaccharide is a commercially available product of Xi'an Wanfang Biotechnology Co., Ltd., which is a water-soluble powder of 90% purity; interleukin- 7 is feline interleukin-7 expressed by Shanghai Bioe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com