Antibacterial coating with pH monitoring function, functional material of antibacterial coating with pH monitoring function and preparation method thereof
A technology of antibacterial coating and functional materials, applied in the fields of botanical equipment and methods, chemicals for biological control, biocides, etc. problems to achieve a strong bonding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] The present invention also provides a kind of preparation method of the antibacterial coating with pH monitoring function, comprises the following steps:
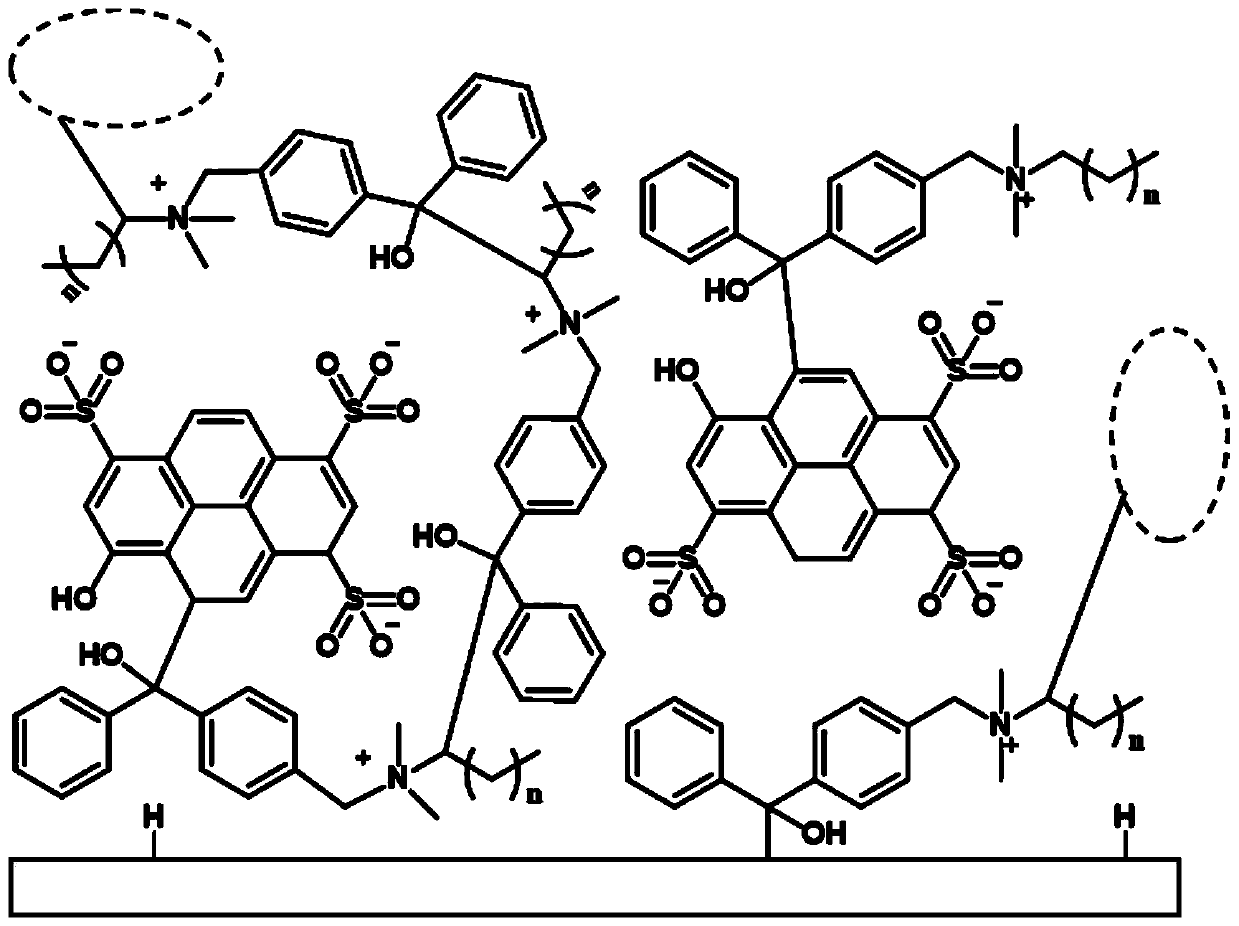
[0092] 1) After mixing 8-hydroxyl-1,3,6-pyrenetrisulfonic acid trisodium salt with a hydrogen-absorbing quaternary ammonium salt solution having a structure of formula (I), a hydrogen-absorbing quaternary ammonium salt / pyranine complex solution is obtained ;
[0093]
[0094] Among them, R 1 and R 2 each independently selected from -CH 3 、-CH 2 CH 3 ;
[0095] R 3 Alkyl group selected from C6~C18;
[0096] 2) After the hydrogen-absorbing quaternary ammonium salt / pyranine complex solution obtained in the above steps is cured by ultraviolet light, an antibacterial coating with a pH monitoring function is obtained.
[0097] The present invention compares the structure, material and specific parameters of the compound in the above preparation method, specific process parameters, and corresponding optimization ...
Embodiment 1
[0126] Preparation of Antibacterial Compounds with pH Monitoring Function and Coating Sample Surfaces
[0127] A) dissolving pyranine in ultrapure water to prepare a pyranine solution with a concentration of 0.025mmol / ml; N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1- Ammonium bromide was dissolved in ultrapure water to prepare a hydrogen pumping quaternary ammonium salt solution with a concentration of 0.075mmol / ml; the above 20mL pyranine solution was added dropwise to 20ml of N-(4-benzoylbenzyl) -N,N-Dimethyldodecyl-1-ammonium bromide solution. Mix and stir until precipitation occurs, centrifuge and wash the N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1-ammonium bromide / pyranine complex. After the complex was washed with ultrapure water, it was dried.
[0128] B) Dissolving the N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1-ammonium bromide / pyranine complex in ethanol to prepare 0.5g / ml of complex solution; the polymer sample is immersed in the above complex solution, and the solven...
Embodiment 2
[0131] A) dissolving pyranine in ultrapure water to prepare a pyranine solution with a concentration of 0.025mmol / ml; N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1- Ammonium bromide was dissolved in ultrapure water to prepare a hydrogen pumping quaternary ammonium salt solution with a concentration of 0.0725mmol / ml; the above 20mL pyranine solution was added dropwise to 20ml of N-(4-benzoylbenzyl) -N,N-Dimethyldodecyl-1-ammonium bromide solution. Mix and stir until precipitation occurs, centrifuge and wash the N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1-ammonium bromide / pyranine complex. After the complex was washed with ultrapure water, it was dried.
[0132] B) Dissolving the N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1-ammonium bromide / pyranine complex in ethanol to prepare 0.5g / ml of complex solution; the polymer sample is immersed in the above complex solution, and the solvent is evaporated at room temperature to obtain the loaded N-(4-benzoylbenzyl)-N,N-dimethyldodecyl-1 - Sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com