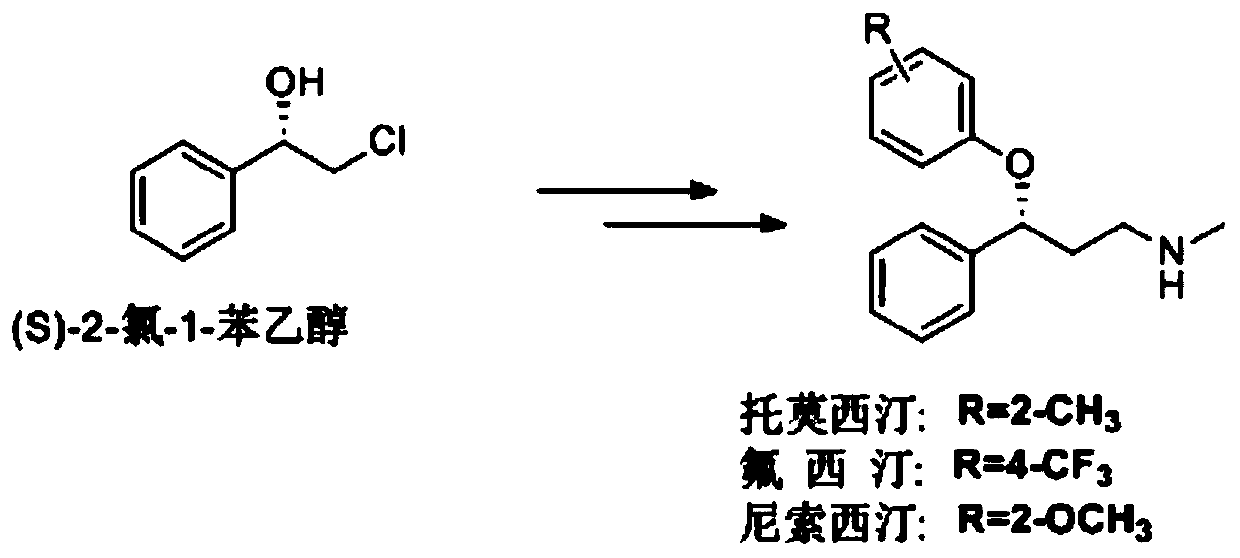
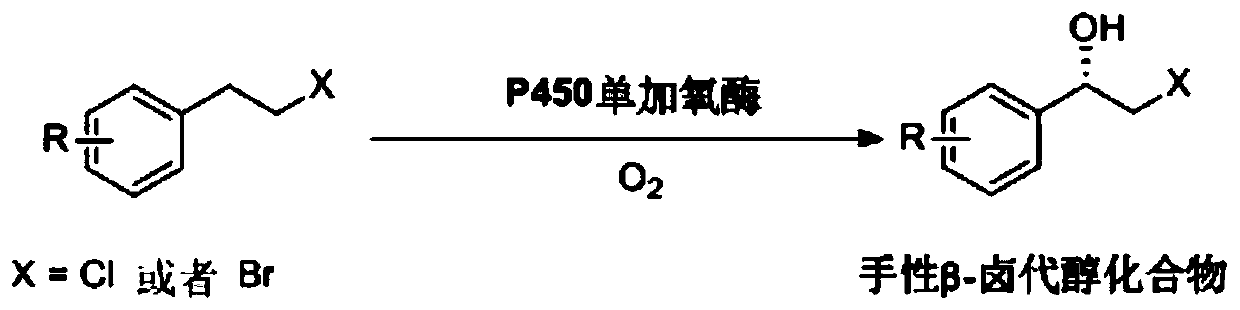
Deinococcus radiodurans-derived P450 monooxygenase mutant and application thereof
A monooxygenase, Deinococcus technology, applied in the biological field, can solve the problems of limited application, low asymmetric hydroxylation activity, low substrate concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Construction of P450 monooxygenase mutation N190D;
[0035] Design random mutation primers P1 (TAATACGACTCACTATAGGG) and P2 (TCGCTTGGAACATTATTAAC) for mutation, extract the plasmid containing the parental P450 gene as a DNA template, and adjust the Mn in the PCR system 2+ Concentration, error-prone PCR. PCR system (50 μL): 25 μL of 2×TaqPCR MaterMix reagent, 1 μL each of P1 and P2 at a concentration of 100 μM, and MnCl at a concentration of 10 μM 2 Solution 1 μL, template plasmid 1 μL, deionized water 21 μL. PCR program: first step, 95°C / 5min; second step, 98°C / 10s, 55°C / 15s, 72°C / 10min, a total of 30 cycles; third step, 72°C / 20min. After the PCR product was purified, the WHOPPCR strategy was used to amplify the whole plasmid, and the obtained PCR product was transformed into Escherichia coli BL21 (DE3) competent cells by heat shock (42°C, 90s). After revived culture (37°C, 1h), spread evenly on LB solid medium containing kanamycin sulfate antibiotic, and ...
Embodiment 2
[0036] Example 2: Construction of P450 monooxygenase mutation N190F;
[0037] In order to obtain a better mutant at position 190, design a site-directed saturation mutation primer P3 for position 190
[0038] (TCATACCATGACCATG NNK AGCCGTCCGCC) and P4 (CGGACGGCT MNN CATGGTCATGGTATGATGC), extract the whole plasmid of mutant N190D as DNA template, and carry out site-directed saturation mutation PCR. PCR system (50 μL): 10 μL of 5×Plusbuffer reagent, 2 μL each of P1 and P2 at a concentration of 100 μM, 2 μL of template plasmid, 4 μL of dNTP reagent, 0.5 μL of PrimeSTAR DNA polymerase, and 29.5 μL of deionized water. PCR program: first step, 95°C / 5min; second step, 98°C / 10s, 60°C / 5s, 72°C / 10min, a total of 30 cycles; third step, 72°C / 20min. After the PCR product was digested with DpnI, it was directly transformed into E. coli BL21 (DE3) competent cells by heat shock (42°C, 90s). After revived culture (37°C, 1h), spread evenly on LB solid medium containing kanamycin sulfate an...
Embodiment 3
[0039] Example 3: Construction of P450 monooxygenase mutation N190F / V356L / A486E;
[0040] In order to obtain a P450 monooxygenase mutant with higher activity, the next round of protein molecular transformation was carried out on the basis of the P450 monooxygenase mutant D190F. Extract the plasmid of P450 monooxygenase mutant D190F as DNA template, use P1 (TAATACGACTCACTATAGGG) and P2 (TCGCTTGGAACATTATTAAC) as random mutation primers, and adjust the Mn in the PCR system 2+ Concentration, error-prone PCR. PCR system (50 μL): 25 μL of 2×TaqPCR MaterMix reagent, 1 μL of P1 and P2 at a concentration of 100 μM, MnCl at a concentration of 10 μM 2 Solution 1 μL, template plasmid 1 μL, deionized water 21 μL. PCR program: first step, 95°C / 5min; second step, 98°C / 10s, 55°C / 15s, 72°C / 10min, a total of 30 cycles; third step, 72°C / 20min. After the PCR product was purified, the WHOPPCR strategy was used to amplify the whole plasmid, and the obtained PCR product was transformed into Esche...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com