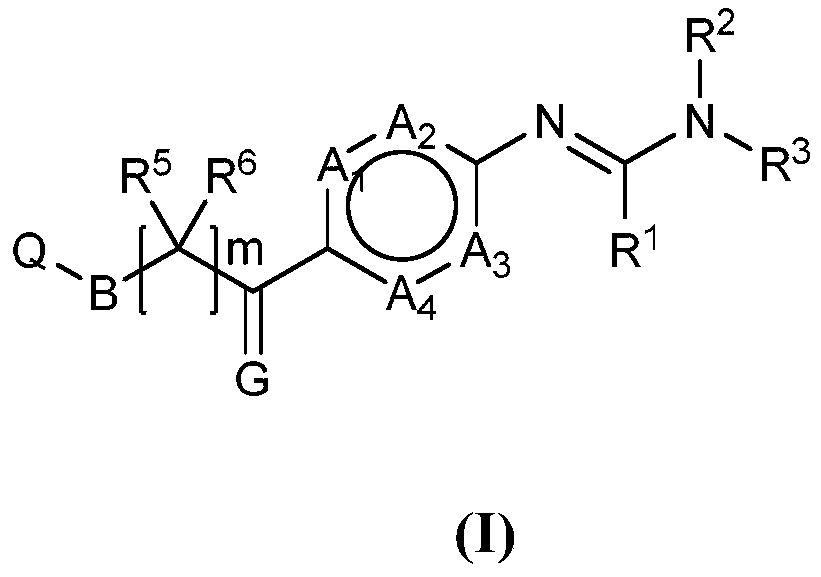
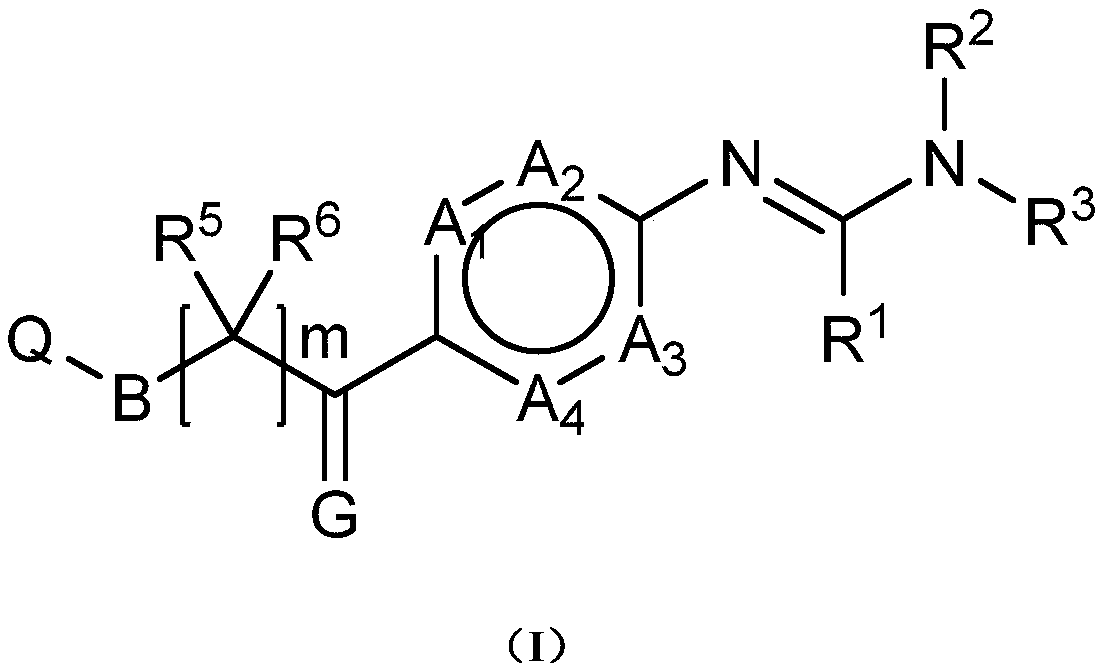
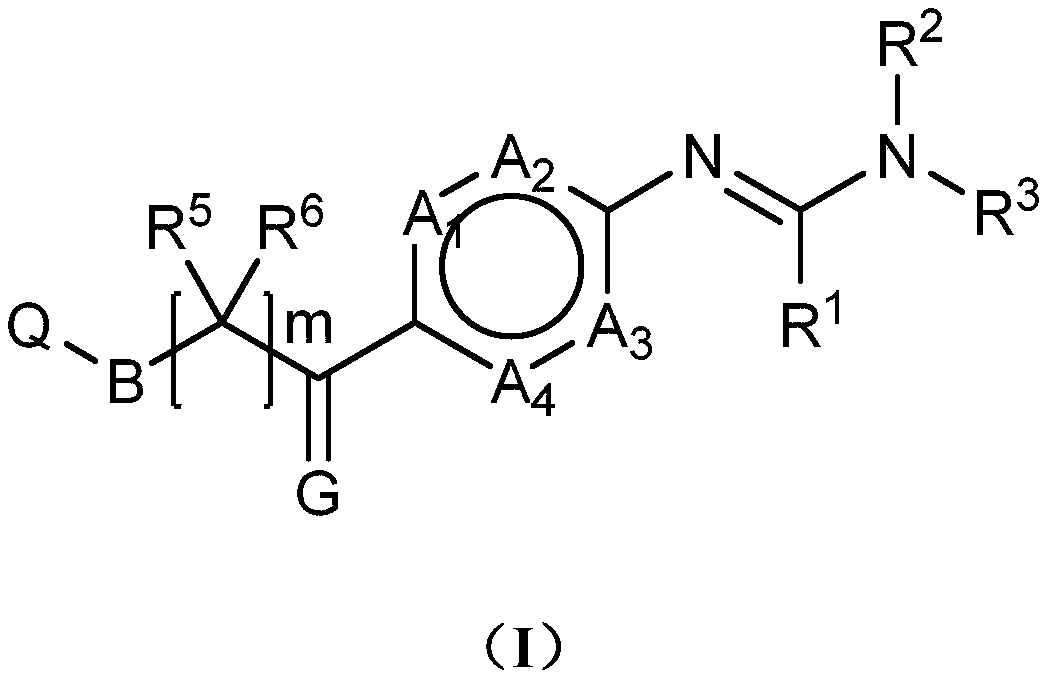
Novel phenylamine compounds
A compound, selected technology, applied in the field of compounds, can solve problems that need to be improved, and achieve the effect of enhancing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0197] Tank mix compositions are generally prepared by diluting one or more premix compositions containing different insecticides with any other adjuvants in a solvent such as water. Suitable carriers and adjuvants can be solid or liquid, substances commonly used in formulation technology, such as natural minerals or regenerated minerals, solvents, dispersants, wetting agents, tackifiers, thickeners, binders or fertilizers .
[0198] Generally, the content of ingredients required in tank mix preparations for foliar or soil application is 0.1-20%, especially 0.1-15%; the content of solid or liquid adjuvants (including solvents, such as water) is 99.9-80%, especially 99.9% to 85%. Wherein the adjuvant may be a surfactant based on the tank mix, with a content of 0-20%, especially 0.1-15%. Usually, the content of required ingredients in the premix preparation for foliar application is 0.1-99.9%, especially 1-95%, and the content of solid or liquid adjuvant (including solvent, su...
example 1
[0402] Example 1: Preparation of N-ethyl-N'-(4-(2-((3-fluorophenyl)thio)acetyl)-2,5-dimethylphenyl)-N-methylimine imine amide
[0403] The preparation of step A N-(2,5-dimethylphenyl) acetamide
[0404] To a stirred solution of 2,5-dimethylaniline (50 g, 413 mmol) in dichloromethane (500 ml) was added triethylamine (144 ml, 1031 mmol) and acetyl chloride (35.2 ml, 495 mmol) at 0°C. The reaction mixture was stirred at 0 °C for 2 hours. After the reaction was complete, the reaction mixture was poured into water. The aqueous layer was extracted with dichloromethane (3 x 1000 mL). The combined dichloromethane layers were separated and washed with water (2 x 500 mL) and brine solution (1 x 250 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered and evaporated under high vacuum to afford the desired N-(2,5-dimethylphenyl)acetamide (60 g, 368 mmol, 89% yield). LCMS (M+H): 165.50
[0405] Step B: Preparation of N-(4-(2-chloroacetyl)-2,5-dimethylph...
example 3
[0413] Example 3: N'-(4-(2-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)thio)acetyl)-2,5-dimethylphenyl)- Preparation of N-ethyl-N-methylformamidine
[0414] Perform steps A-D according to the procedure described in the general scheme.
[0415]To 1-(4-amino-2,5-dimethylphenyl)-2-((3-chloro-5-(trifluoromethyl)pyridin-2-yl)thio)ethan-1-one ( To a stirred solution of 0.25 g, 0.667 mmol) in trimethyl orthoformate (10 mL), p-toluenesulfonic acid hydrate (6.34 mg, 0.033 mmol) was added, and the resulting mixture was stirred at 105° C. for 2 h. After complete conversion of the aniline derivative, the volatiles were evaporated to give the intermediate. This intermediate was dissolved in 1,4-dioxane (10ml), N-ethylmethylamine (0.394g, 6.67mmol) was added, and the mixture was stirred at 105°C for 2 hours. After completion of the reaction, the reaction mixture was evaporated to give the crude compound, which was purified by preparative HPLC to give the desired N'-(4-(2-((3-chloro-5-(tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com