Novel phosphoinositide 3-kinase inhibitor and preparation method and application thereof
A solvate, independent technology, applied in the field of medicinal chemistry, can solve the problem of few reports of PI3Kγ inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0437] The present invention provides the preparation method of above-mentioned formula I compound, it comprises:
[0438] 1) The preparation method of formula IA compound, concrete steps are as follows:
[0439]
[0440] S1: Replace X in compound IA-1-1 with R 3 (preferably by coupling reaction or substitution reaction), to obtain compound IA-1-2;
[0441] S2: Compound IA-1-2 is reacted with compound IA-a (preferably by condensation reaction) to obtain compound IA-1-3;
[0442] S3: Compound IA-1-3 is reacted with compound IA-b (preferably in the presence of Grignard reagent and metal organic compound, more preferably in the presence of isopropylmagnesium chloride and n-butyllithium), to obtain compound IA-1 -4;
[0443] S4: Compound IA-1-4 is reacted with compound IA-c (preferably by condensation reaction) to obtain the compound of formula IA;
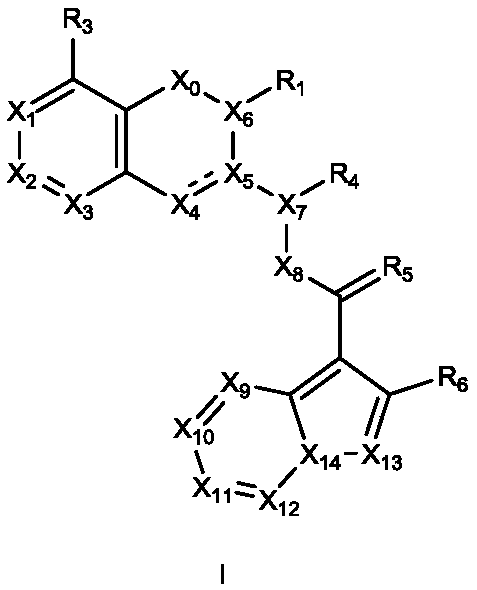
[0444] Wherein: X is chlorine, bromine or iodine; X 4 for CH or CR 7 ;X 1 、X 2 、X 3 、X 6 、X 8 、X 9 、X 10 、X 11 、X 12...
Embodiment 1
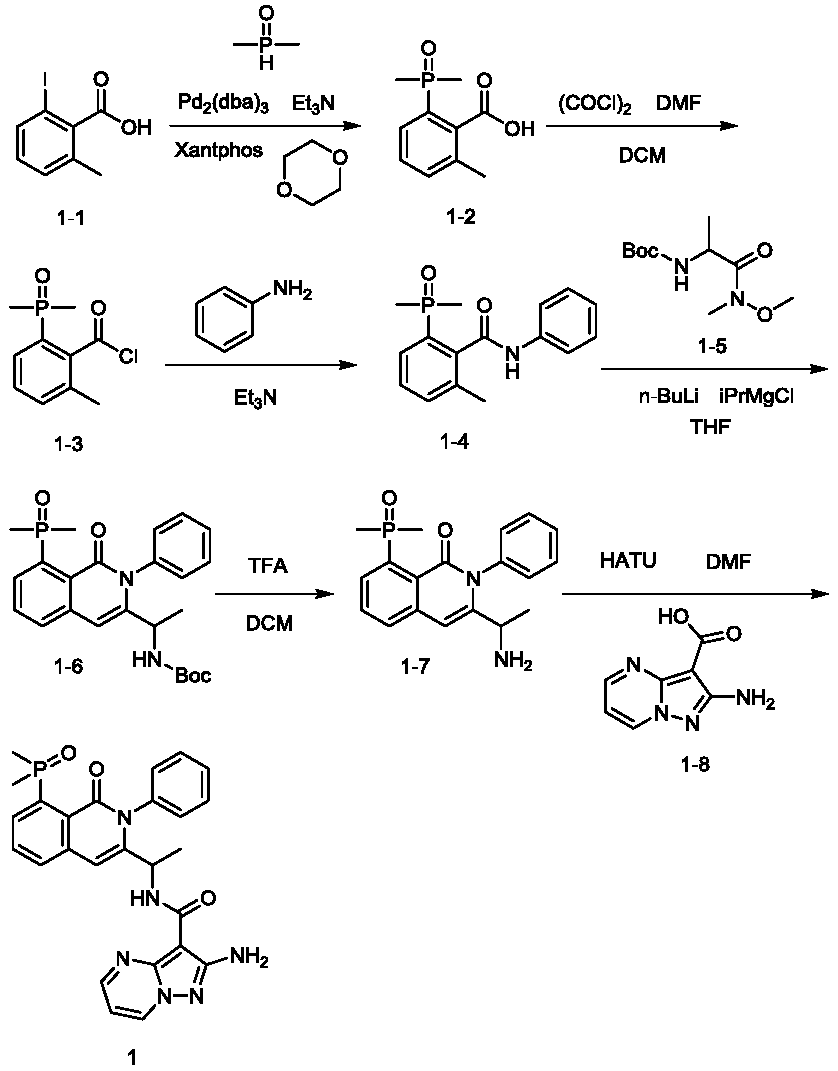
[0540] Example 1: Synthesis of Compounds 1-3.
[0541] S1: in N 2 Under protective conditions, dimethylphosphine oxide (1.25mmol, 1eq), 2-iodo-6-methylbenzoic acid (compound 1-1) (1.35mmol, 1.08eq), triethylamine were added successively to the reaction vessel (1.44mmol, 1.15eq) and 1,4-dioxane (1.5mL), then add Pd 2 (dba) 3 (4mmol, containing 8μmol of Pd) and the bisphosphine ligand Xantphos (8μmol), the solution was stirred at 80°C for 2h, the reaction was complete by TLC, filtered, and separated by silica gel column chromatography to obtain compound 1-2. ESI-MS: m / z 213.06, [M+H] + .
[0542] S2: To a stirred mixture of compound 1-2 (1.5 mmol, 1 eq) and N,N-dimethylformamide (0.2 mL) in dichloromethane (12 mL) was added oxalyl chloride (1.65 mL) within 5 min at room temperature mmol, 1.1 eq), and stirred at room temperature for 2 h, the mixture was concentrated in vacuo, and the concentrated residue (containing compound 1-3) was dissolved in dichloromethane (15 mL), and...
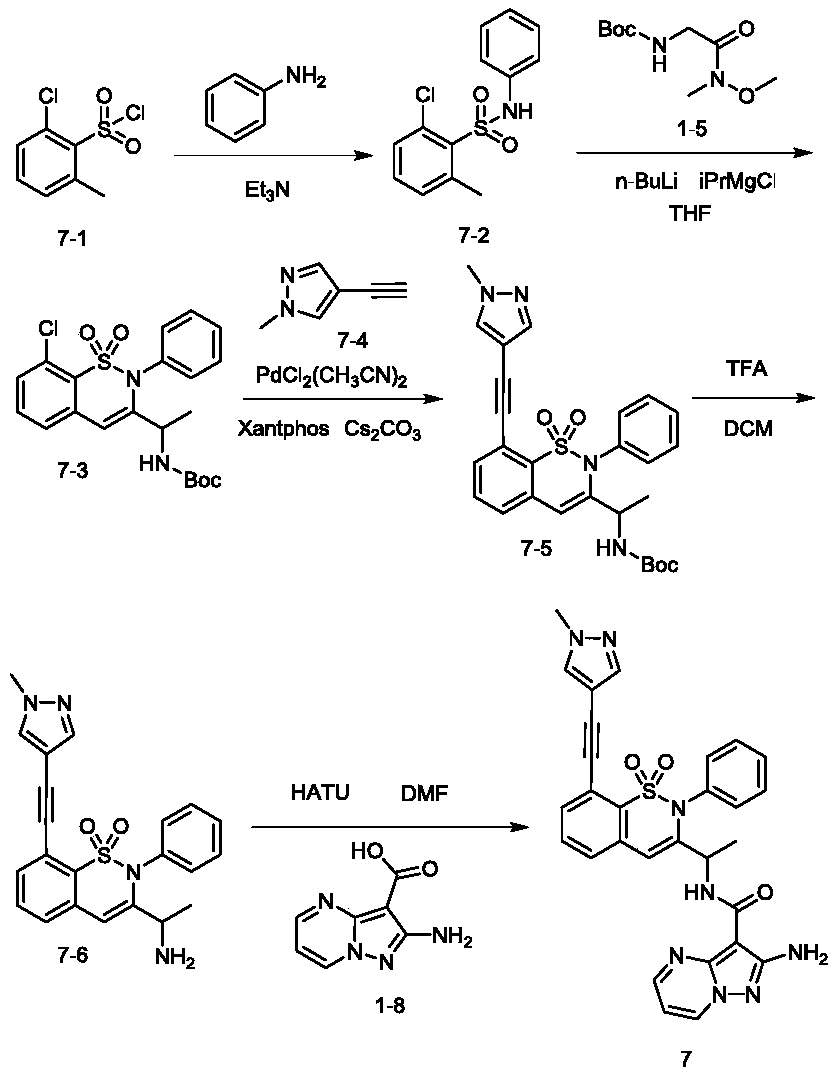
Embodiment 2
[0550] Example 2: Synthesis of Compounds 4-6.
[0551] Referring to Example 1, compound 1-2 is replaced by the following compounds:
[0552] The synthetic process of compound 4 is similar to compound 1, and the difference is that 2-dimethylphosphoryl-6-methylbenzoic acid (compound 1-2) in the synthetic process of compound 1 is replaced by 2-methyl-6- (Pentafluoro-λ 6 -sulfanyl)benzoic acid to obtain compound 4. ESI-MS: m / z 551.12, [M+H] + .
[0553] The synthetic process of compound 5 is similar to that of compound 4, except that tert-butyl 1-(methoxy(methyl)amino)-1-oxopropan-2-ylcarbamate is replaced by (R)-1-(methyl tert-butyl oxy(methyl)amino)-1-oxopropan-2-ylcarbamate.
[0554] The synthetic process of compound 6 is similar to compound 4, the difference is that tert-butyl 1-(methoxy(methyl)amino)-1-oxopropan-2-ylcarbamate is replaced by (S)-1-(methyl tert-butyl oxy(methyl)amino)-1-oxopropan-2-ylcarbamate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com