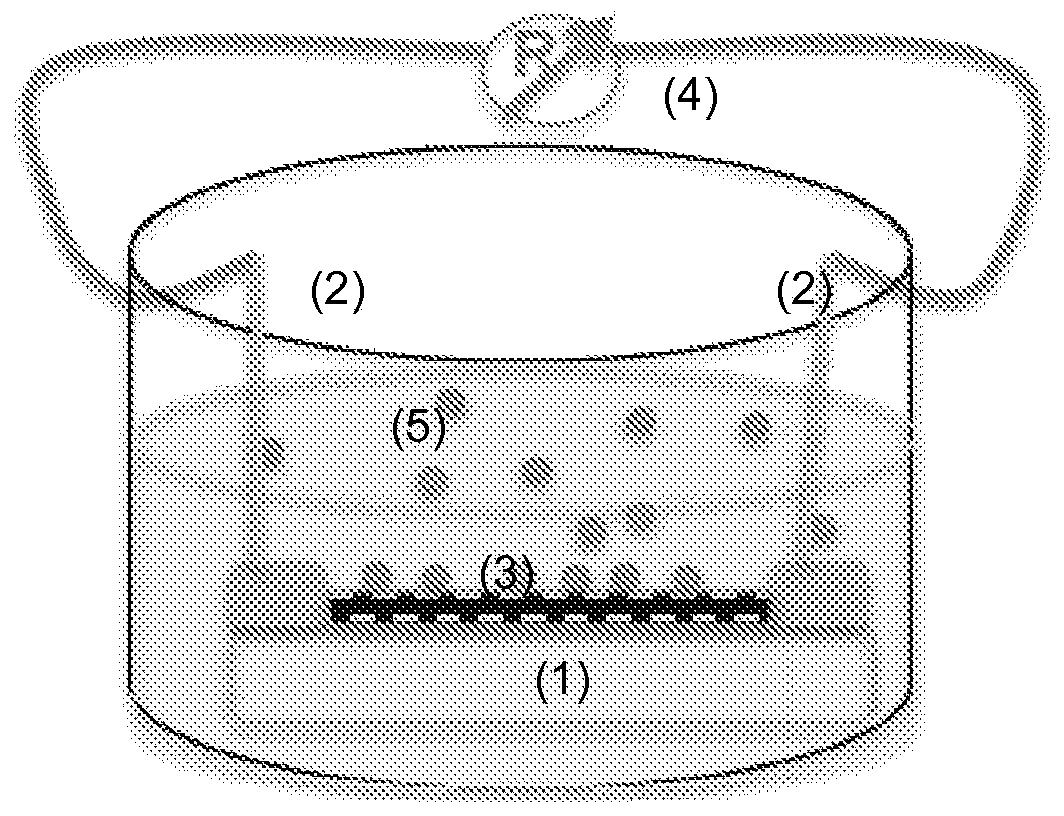
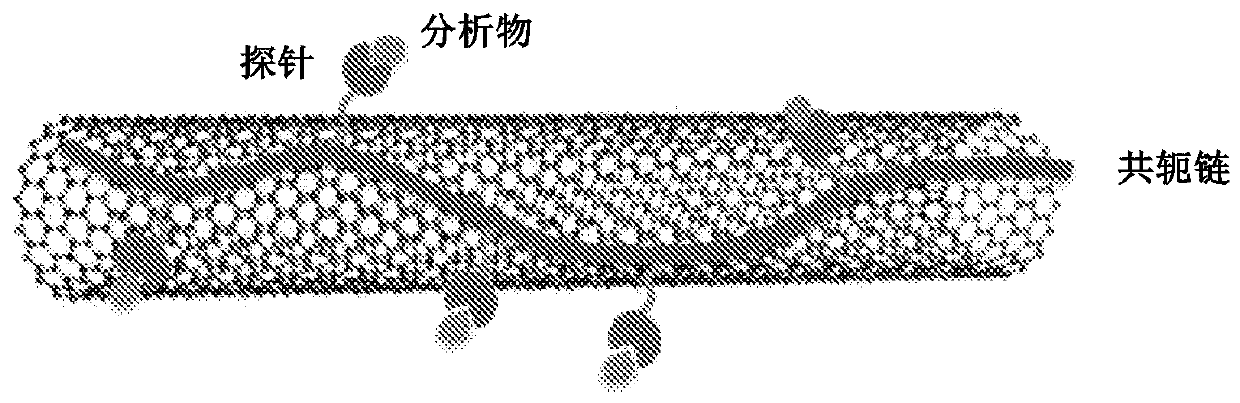
Chemical sensors based on carbon NANO tubes functionalised by conjugated polymers for analysis in aqueous medium
A technology of conjugated polymers and carbon nanotubes, applied in the fields of micro chemical sensors, selective quantification, and aqueous solution analysis, can solve the problem that chemical sensors cannot fully meet the requirements of reliability, rapidity, selectivity, sensitivity, and portability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Example 1: Synthesis of Polymers of Formula 8 for Detection and / or Quantification of Hypochlorite Ions
[0171] The compound of formula 1 in the reaction scheme below was synthesized as described in Photochemical and Photobiological Sciences, 2013, 12, 284-297. The compound of formula 2 was synthesized as described in Journal of Chemical Sciences, 2015, 127, 383-394.
[0172]
[0173] The compound of formula 7 was synthesized from compound 2 as follows. Compound 2 (850mg; 0.15mmol), 2,2'-(9,9-dihexyl-9H-fluorene-2,7-diyl)bis(1,3,2-dioxaborinane) (500g ; 0.85mmol) and catalyst Pd (PPh 3 ) 4 (50 mg; 0.04 mmol) was dissolved in 30 mL of degassed toluene. 6 mL of aqueous potassium carbonate solution (590 mg; 4.27 mmol) was added. The reaction medium is heated to reflux for 3 days under an inert atmosphere. 9 μL (0.085 mmol) of bromobenzene was added to remove the terminal boroaryl ester functional group; after stirring for 6 hours, 10 mg (0.085 mmol) of phenylboron...
Embodiment 2
[0178] Embodiment 2: synthesis is used for detecting and / or the polymkeric substance of the formula 9 of quantitative chloride ion
[0179] Compound 3 in the reaction scheme below was synthesized as described in Macromolecules, 2005, 38, 745-751: Aniline was added to a mixture of 2,7-dibromo-9-fluorenone and aniline hydrochloride in an argon atmosphere. The reaction medium is heated to 150° C. for 6 hours and then cooled to ambient temperature. The reaction medium is poured into water and extracted with ethyl acetate. Compound 3 was obtained after recrystallization in hexane.
[0180] Compound 4 in the reaction scheme below was synthesized as follows. In an argon atmosphere, 0.22 mL (1.9 mmol) of phenylisocyanate was added dropwise to a solution of 400 mg (0.8 mmol) of compound 3 in 10 mL of dichloromethane. The reaction mixture was stirred at ambient temperature for 4 hours, then 20 mL of ether was added. Compound 4 (410 mg, 69% yield) was obtained as a gray solid after f...
Embodiment 3
[0185] Example 3: Synthesis for joint detection and / or quantification of Ca 2+ and Mg 2+ Ionic polymer of formula 10
[0186] Compound 5 in the Reaction Scheme below was synthesized from Compound 3 in the Reaction Scheme below as follows. 124 mg (0.9 mmol) potassium carbonate and 15 mg (0.09 mmol) potassium iodide were added to a solution containing 500 mg (1 mmol) of compound 3 in THF at ambient temperature. Then 1.38 g (9 mmol) methyl bromoacetate were added slowly. The reaction medium is stirred at 100° C. for 18 hours and then poured into water. The precipitate was filtered and dried. 596 mg (0.75 mmol) of the compound were obtained in the form of a pale orange solid.
[0187] 1 H NMR (300MHz, CDCl 3 ):δ7.54-7.51(d,2H),7.44-7.41(d,4H),6.98-6.96(d,4H),6.47-6.44(t,4H),4.10(s,4H),3.74(s ,12H).
[0188]
[0189] Compound 6 of the above reaction scheme was synthesized as follows. 2 ml (5 mmol) of aqueous sodium hydroxide solution was slowly added to a solution of 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com