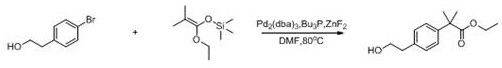
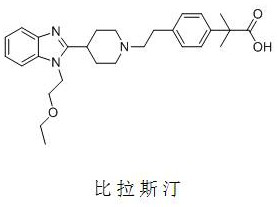
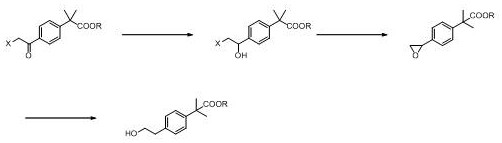
A kind of synthetic method of bilastine intermediate
A technology of bilastine and a synthesis method, applied in the field of drug synthesis, can solve the problems of complex reaction epoxidation synthesis, difficulty in industrial scale-up, difficulty in purification and the like, and achieves low cost of raw materials, simple requirements for equipment and experimental conditions, and high application value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] The present invention will be further described below in conjunction with specific embodiments. Of course, the embodiments described below are only a part of the present invention, not all embodiments.
[0034] Synthesis of intermediate compound B: In a 500ml three-neck flask, weigh phenethyl acetate (65.6g, 0.4mol), add 100ml of dichloromethane and stir. Cool down to 0°C, add anhydrous aluminum trichloride (51.1g, 0.48mol) and stir, add dropwise isobutyryl chloride (42.5g, 0.4mol), react for 1 hour, return to room temperature (25°C) and stir for 4 hours. After the reaction was completed, dilute hydrochloric acid was added to quench the reaction, dichloromethane was added to extract twice, the organic phases were combined, and the organic phases were washed once with water and saturated brine, evaporated to dryness and purified to obtain intermediate compound B (74.9g, y =80%).
[0035] 1HNMR (400Hz, CDCl 3 ( s, 3H), 1.21-1.22 (d, 6H).
[0036] Synthesis of intermed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com