Preparation method of cyclen
A technology for the preparation of cylindrine and its intermediates, which can solve the problems of easy decomposition, many side reactions, and unfavorable scale-up production, and achieve good reaction selectivity, reduced production costs, and high reaction yield. Considerable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
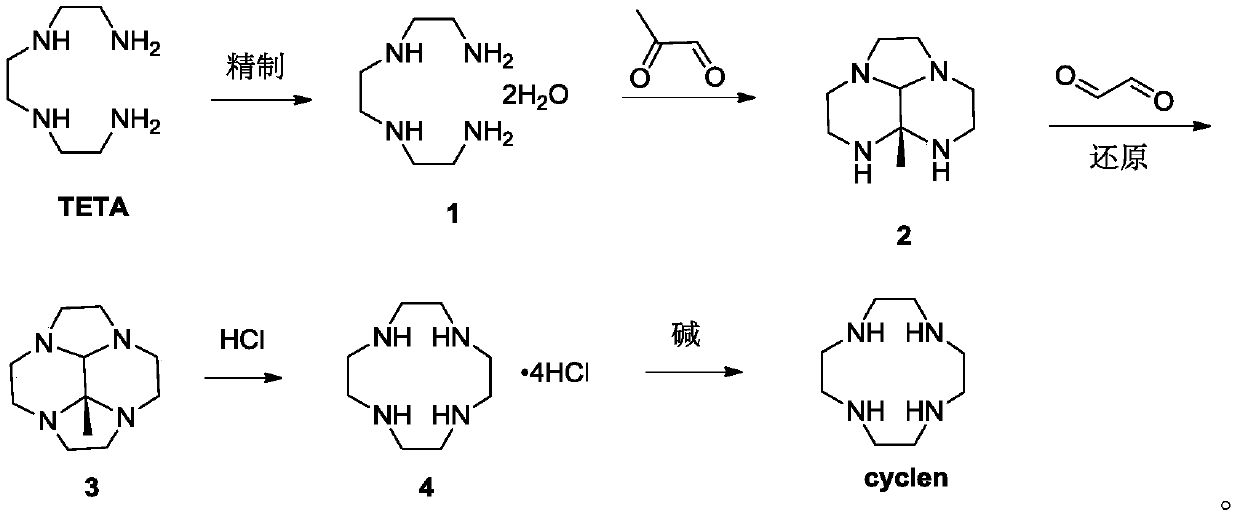
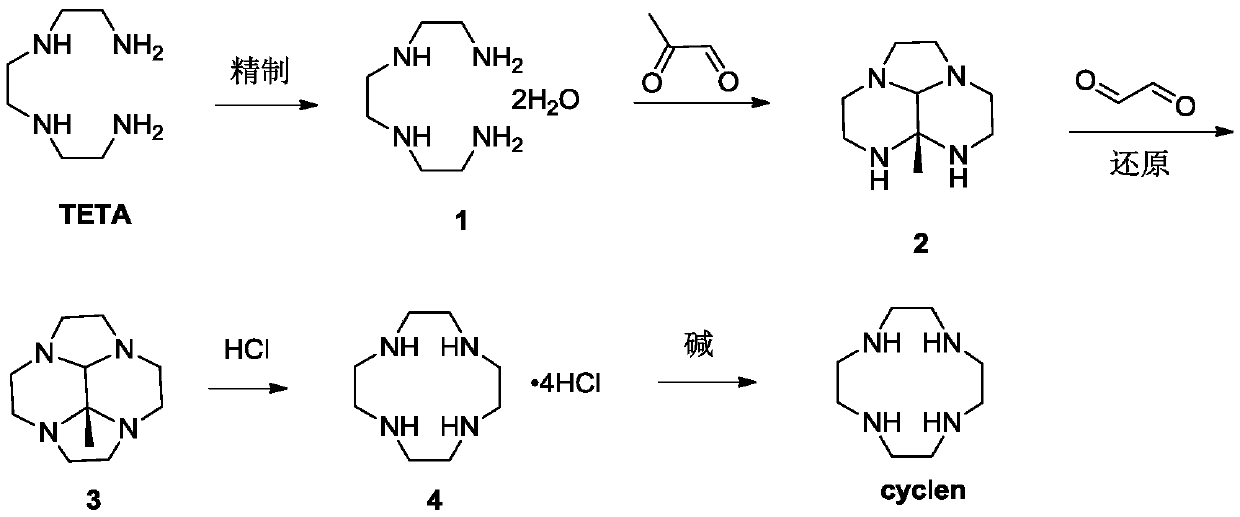
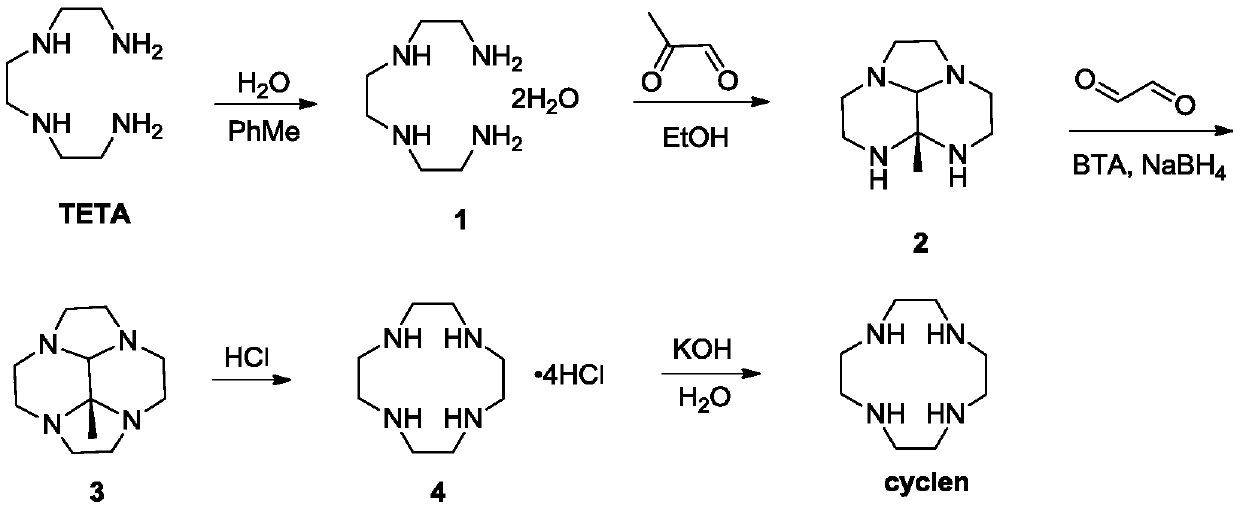
[0045] Step 1: Refining of TETA
[0046]
[0047] Add 1.0kg of triethylenetetramine (TETA) and 0.17kg of water into the reaction vessel, control the temperature at 20-50°C, add a small amount of seed crystals, then add 1.5-2.0kg of toluene dropwise, and control the temperature at 20-50°C, drop After completion, lower the temperature to 0-20° C., and keep stirring for 2-4 hours, then filter, collect the filter cake, and obtain 850 g after drying, with a purity of 99%.
[0048] 1 H-NMR (400MHz, CDCl 3 ,ppm)δ:2.92(s,4H),2.56(m,12H),2.49(m,6H).
[0049] The second step: the preparation of intermediate 2
[0050]
[0051] Add 350g of compound 1 into the reaction vessel, add 3~4L of absolute ethanol, cool down to -10~5°C, under nitrogen atmosphere, add dropwise a solution of 40% aceguvaldehyde (347~520g) in ethanol (1.5~2.0L), keep warm After stirring for 2-4 hours, TLC monitored the completion of the reaction, and the product was directly used in the next reaction without...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com