A new synthesis method of multi-substituted 4,5,6,7-tetrahydrobenzofuran
A technology of benzofuran and synthesis method, applied in the field of organic chemical synthesis, can solve the problems of residual trace transition metals, low total yield and the like, and achieve the effect of wide applicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
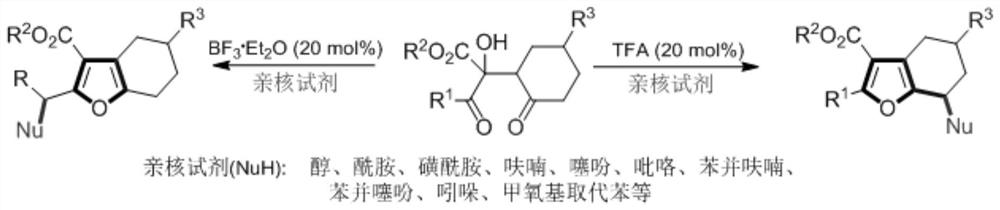
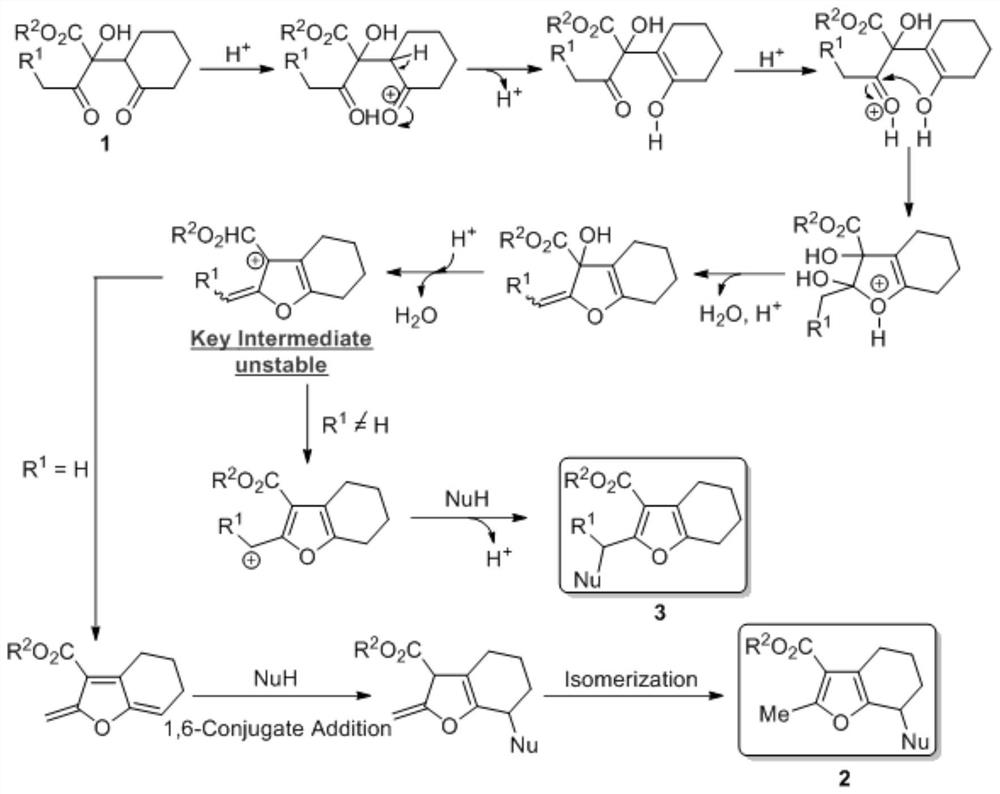
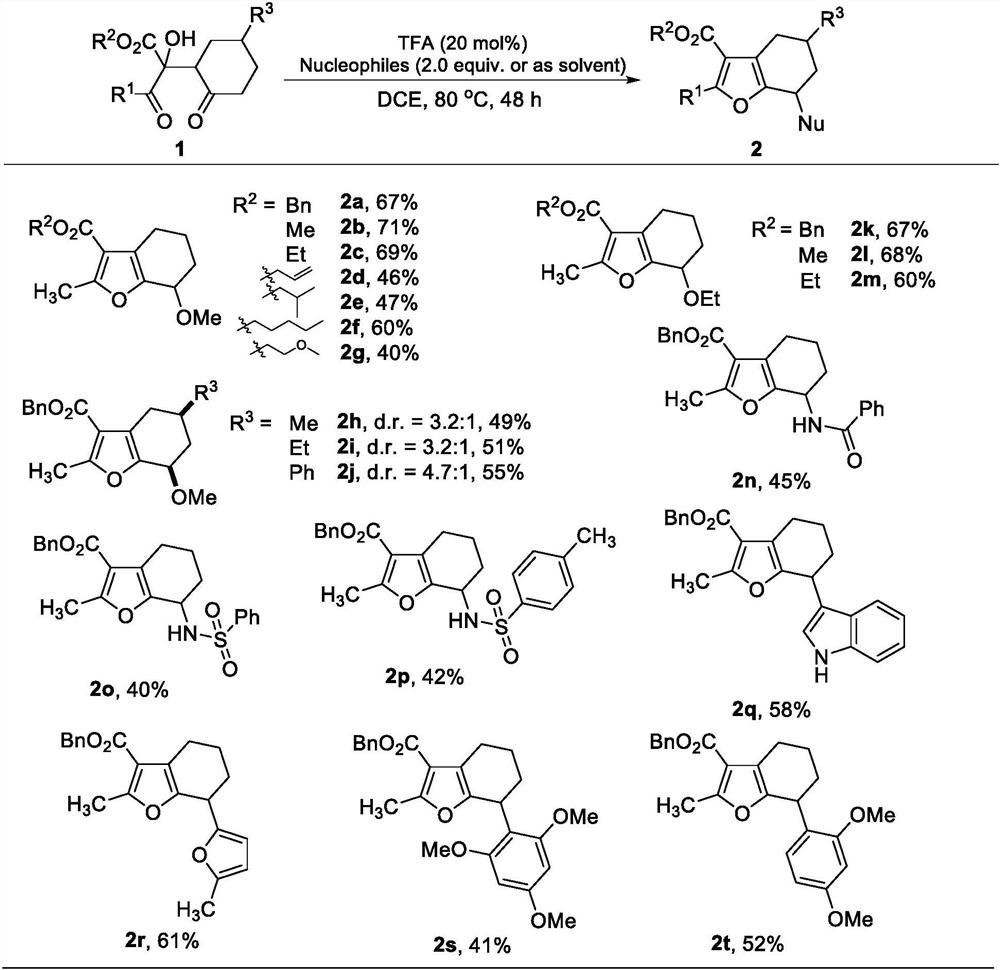
[0015] Synthesis of 2,3,7-trisubstituted-4,5,6,7-tetrahydrobenzofuran: Add 2-hydroxyl-1, 4-diketone (1,0.2mmol), 1,2-dichloroethane (2.0mL), nucleophile (0.4mmol), 2,2,2-trifluoroacetic acid (0.04mmol), thereafter four Vinyl fluoride plug, the pressure tube was stirred in an oil bath at 80°C for 48 hours, after which the reaction was stopped, the reaction liquid was concentrated, and the product 2 was obtained by column chromatography. Note: When methanol or ethanol is used as the reaction reagent, directly use 2 mL of methanol or ethanol as the reaction solvent without using 1,2-dichloroethane.
[0016] The range of substrates used in this reaction is very wide. Table 1 shows some suitable substrates. Alcohols, amides, furans, indole and other charge-rich substrates can all participate in the reaction. TFA in Table 1 refers to 2,2,2-trifluoroacetic acid, Nucleophiles refers to various types of rich substrates, DCE refers to 1,2-dichloroethane, "or as solvent" means "or use a...
Embodiment 2
[0020] Synthesis of 2,3-disubstituted 4,5,6,7-tetrahydrobenzofuran: Add 2-hydroxy-1,4-di - Ketone (1,0.2mmol), 1,2-dichloroethane (2.0mL), nucleophile (0.4mmol), boron trifluoride ether (0.04mmol). Thereafter, a tetrafluoroethylene stopper was plugged, and the pressure-resistant tube was stirred in an oil bath at 90° C. for 24 hours. Thereafter, the reaction was stopped, the reaction solution was concentrated, and the product 3 was obtained by column chromatography. Note: When methanol or ethanol is used as the reaction reagent, directly use 2 mL of methanol or ethanol as the reaction solvent without using 1,2-dichloroethane.
[0021] The range of substrates used in this reaction is very wide. Table 2 shows some suitable substrates. Alcohols, amides, furans, thiophenes, benzofurans, benzothiophenes, indole and other charge-rich substrates can all participate in the reaction. In Table 2, Nucleophiles refer to various types of electro-rich substrates, DCE refers to 1,2-dichlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com