Compositions useful in treatment of spinal muscular atrophy
A technology of identity and protein, applied in drug combination, introduction of foreign genetic material using carrier, application, etc., can solve problems such as small improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0226] Example 1-New clade FAAV-AAVhu68
[0227] A QIAamp column (Qiagen) was used to extract tissue DNA from human tissue samples as a PCR template according to the manufacturer's recommendation (with the following modifications). As described by Gao et al. [Proc Natl Acad Sci USA, 2002 Sep3, 99(18): 11854-11859 (Epub August 21, 2002)], because Q5 DNA polymerase ( Hot Start High-Fidelity2X Master Mix, NEB) has extraordinary high fidelity and strong efficiency. The Q5 DNA polymerase was selected to recover the full-length VP1 gene of potential AAV in the sample, and the primer set was modified as follows: Use primer prm504[GCTGCGTCAACTGGACCAATGAGAAC SEQ ID NO: 23] was used instead of AV1NS, and prm505 [CGCAGAGACCAAAGTTCAACTGAAACGA, SEQ ID NO: 24] was used instead of reverse primer AV 2CAS. The PCR conditions were modified as follows:
[0228] μL water9 prm5041.25 prm5051.25 template1 2XQ512.5
[0229] PCR program
[0230]
[0231] The ~3kb band from PCR was cut out from t...
Embodiment 2
[0234] Example 2-AAVhu68 vector
[0235] Generate and evaluate AAVhu68 and AAV9 vectors carrying multiple tags (such as GFP and LacZ). Each vector is produced in 293 cells using triple transfection technology, such as Gao et al. [Gao, Guang-Ping, et al. "Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy." Proceedings of the National Academy of Sciences 99.18(2002):11854-11859].
[0236] 1. The production of pAAVhu68 trans plasmid
[0237] The nucleic acid sequence encoding vpl capsid protein is provided in SEQ ID NO:7.
[0238] The pAAV2 / hu68 trans plasmid was prepared by loading the VP1 gene of hu68 into the pAAV2 / 9 backbone instead of the AAV9 VP1 gene to evaluate packaging efficiency, yield and transduction properties. The pAAV2 / 9 plasmid contains the AAV2 5'and 3'ITRs flanking the capsid gene, and is available from Penn Vector Core [University of Pennsylvania, Phila, PAUS, pennvectorcore.med.upenn.edu].
[0239] 2. Production of AAVhu68 vector
...
Embodiment 3
[0247] Example 3-AAV vector containing hSMN
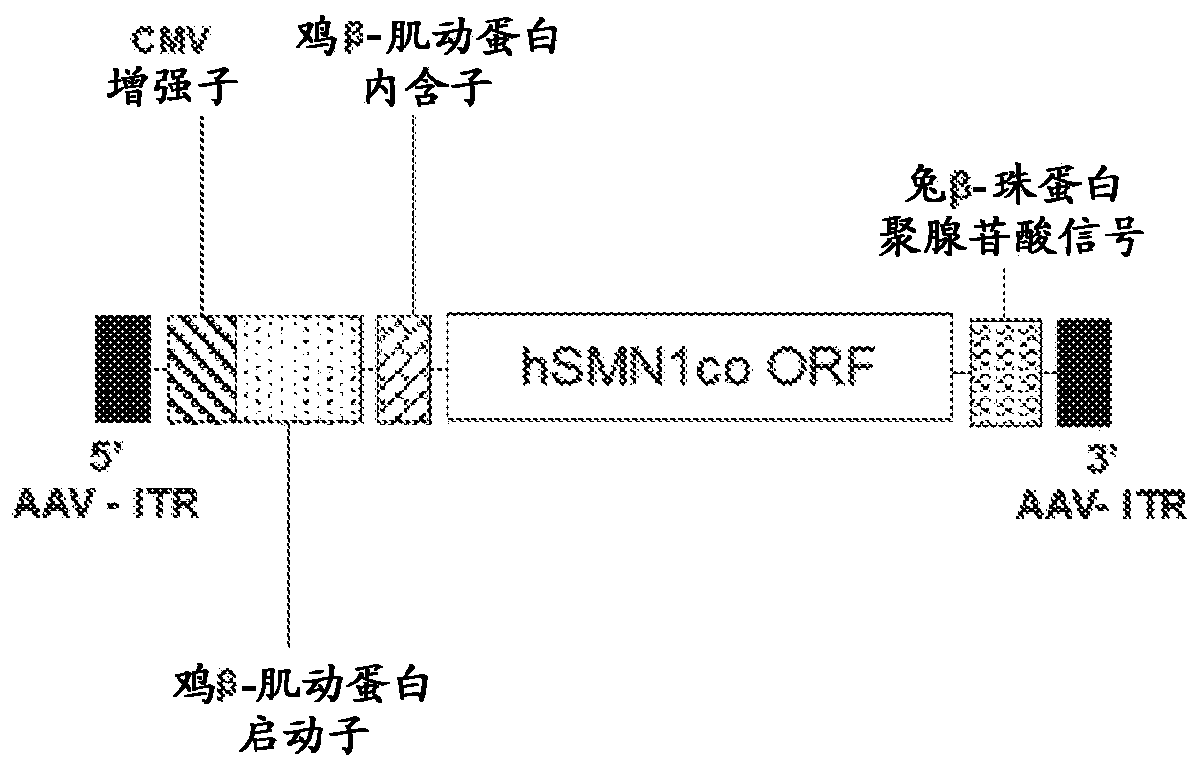
[0248] AAVhu68.CB7.CI.hSMN1co.RBG consists of external components and internal DNA genome. The external vector component is serotype hu68, T=1 icosahedral capsid, which is composed of 60 copies of three AAV viral proteins VP1, VP2 and VP3 at a ratio of about 1:1:8-10. The capsid contains a single-stranded DNA genome composed of a human motor neuron survival 1 (hSMN1) transgene flanked by two AAV inverted terminal repeats (ITR). The enhancer, promoter, intron, hSMN1 coding sequence, and polyadenylation (polyadenylation) signal constitute the human SMN1 transgene. ITR is the genetic element responsible for genome replication and packaging during the vector production process, and is the only viral cis element required to produce rAAV. The expression of the hSMN1 coding sequence is driven by the CB7 promoter, which is a hybrid between the cytomegalovirus (CMV) immediate early enhancer (C4) and the chicken β-actin promoter. The transcr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com