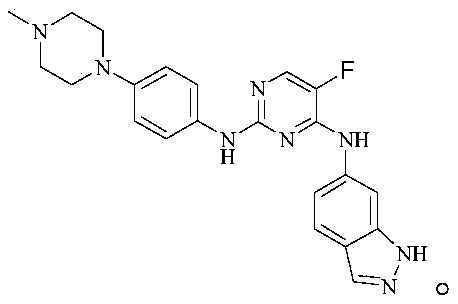
2-N-aryl-4-N-aryl-5-fluoropyrimidine compound as well as preparation method and application thereof
A technology of fluoropyrimidines and compounds, applied in the field of 2-N-aryl-4-N-aryl-5-fluoropyrimidines and their preparation, can solve the problem of unseen small molecule inhibitors and limit the survival time of tumor patients Prolongation and other issues, to achieve strong inhibitory activity, the effect of increased inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] compound synthesis
[0026] The melting point was determined by X-4 micro melting point apparatus (the temperature was not corrected); the mass spectrum was determined by Agilent 1100 series single quadrupole liquid mass spectrometer. 1 H-NMR, 13 C-NMR was measured by Bruker AVANCE III500 nuclear magnetic resonance instrument (DMSO-d 6 as solvent, TMS as internal standard); TLC with GF 254 Silica gel plate and silica gel powder (200-300 mesh) for column chromatography were purchased from Aladdin Reagent Company (aladdin, Shanghai Jingchun Biochemical Technology Co., Ltd.) and Sinopharm Chemical Reagent Co., Ltd.; other reagents and solvents used were commercially available Analytical pure commodity, if necessary, use after anhydrous drying treatment.
[0027] The synthetic route is as follows:
[0028]
[0029] 1.1 Synthesis of intermediates 3a~3j (taking 3a as an example)
[0030] Dissolve 333.94 mg (2.0 mmol) of 2,4-dichloro-5-fluoropyrimidine and 516.96 mg (4...
Embodiment 2
[0039] Example 2 to FGFR4 V550L Protein Kinase Inhibitory Activity Test
[0040] The Caliper EZ Reader drug screening platform was used to test the effect of target compounds 8a-8j on FGFR4 V550L Inhibitory activity of protein kinases. Experimental procedure: configure 1.25x kinase reaction buffer (62.5mmol / L HEPES, pH 7.5; 0.001875% Brij-35; 12.5mmol / LMgCl2; 2.5mM DTT) and kinase reaction termination solution (100mmol / L HEPES, pH 7.5; 0.015 %Brij-35; 0.2% Coating Reagent#3); Add 10 μl of 2.5x FGFR4 to 5 μl of 5x concentration compound solution (dissolved in DMSO and diluted 10 times with water) V550L Kinase solution (add kinase in 1.25x kinase reaction buffer), incubate at room temperature for 10 min, then add 10 μl of 2.5x substrate peptide solution (add FAM-labeled peptide and ATP in 1.25x kinase reaction buffer), at 28°C Add 25 μl kinase reaction stop solution after reacting for a specific time. Test and collect data on Caliper, inhibition rate of kinase activity=(max-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com