Organic compound based on nitrogen-containing heterocyclic ring and preparation method and application of organic compound
A technology of organic compounds and nitrogen heterocycles, applied in the field of OLED materials, can solve the problems of low lifetime and low luminous efficiency, and achieve the effects of high structural regularity, high luminous efficiency, and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
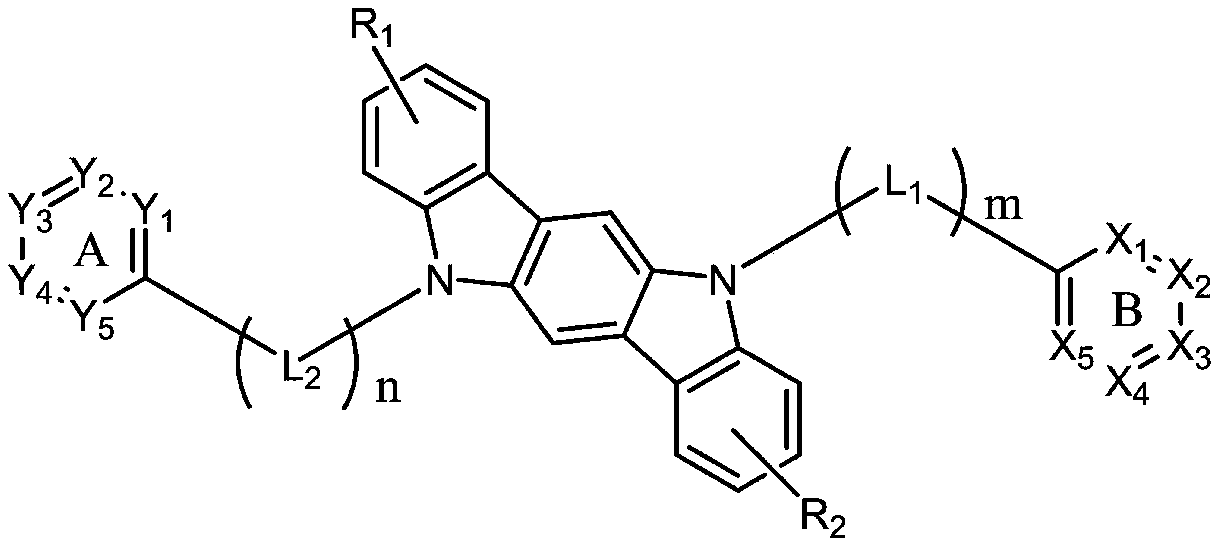
[0032]
[0033] The synthetic method of organic compound (1) is as follows:
[0034]
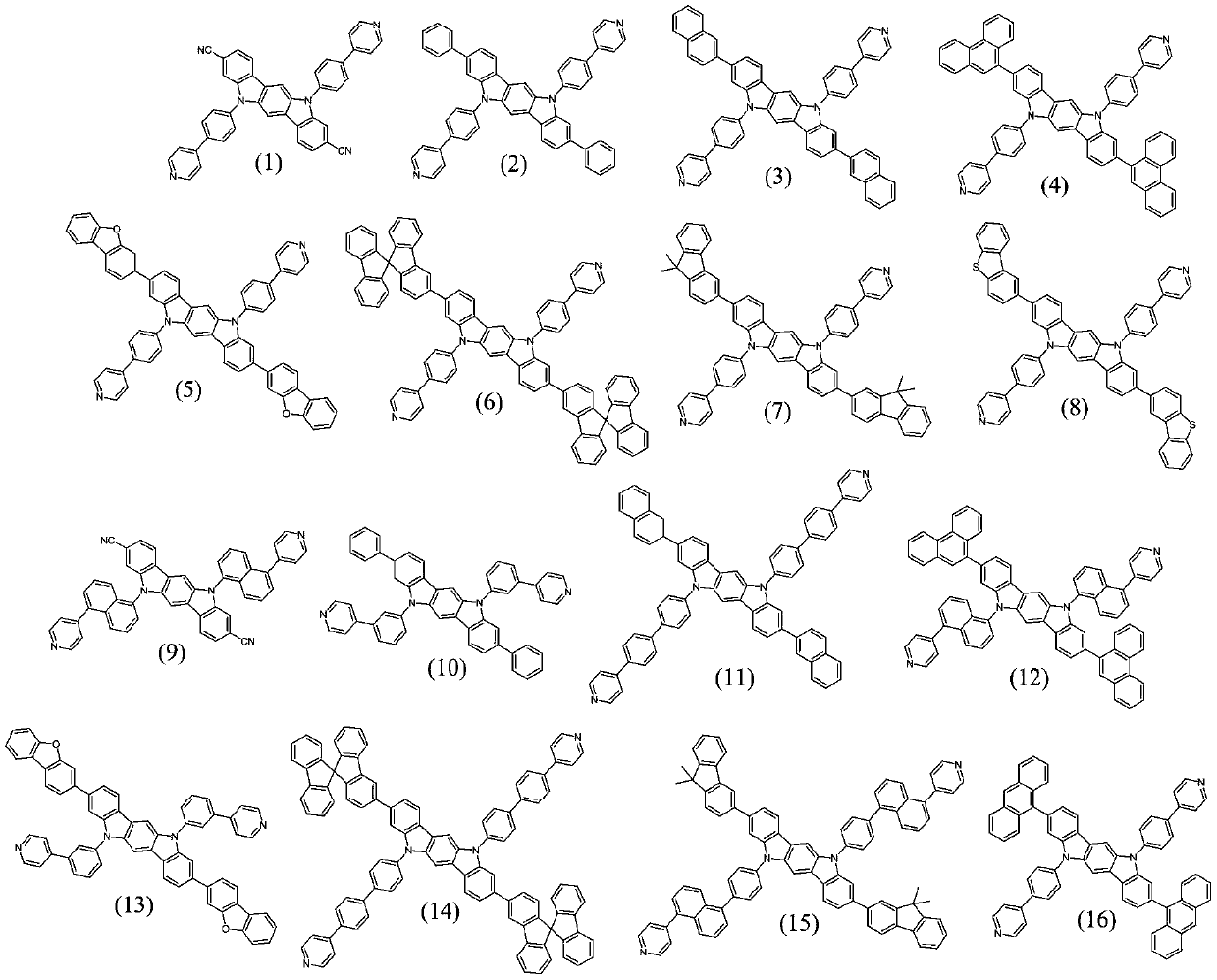
[0035] Compound 1-a (5g, 613.97g / mol, 8.14mmol), compound 1-b (2.1eq, 2.1g, 123.05g / mol, 17.1mmol) and sodium carbonate (4eq, 3.45g, 105.99g / mol, 32.57mmol) was added to a mixed solvent composed of toluene (100g, 20 times the mass of compound 1-a) and water (100g, 20 times the mass of compound 1-a), stirred and mixed and then added three (o-toluene Base) phosphine (5%eq, 0.12g, 304.37g / mol, 0.41mmol) and palladium (II) acetate (1%eq, 0.02g, 224.51g / mol, 0.08mmol), warming up to reflux and stirring for 10h, the reaction After finishing cooling to room temperature, filter, separate the toluene, extract the aqueous phase with toluene, combine the organic phases, wash with water, dry and then concentrate under reduced pressure to obtain the crude product, which is purified by column chromatography to obtain the organic compound (1) (4.04g , yield 81.1%), MS (EI): 613.2 (M+H) + .
Embodiment 2
[0037]
[0038] The synthetic method of organic compound (4) is as follows:
[0039]
[0040] Compound 2-a (5g, 916.11g / mol, 5.46mmol), compound 2-b (2.1eq, 1.41g, 123.05g / mol, 11.46mmol) and sodium carbonate (4eq, 2.31g, 105.99g / mol, 21.83mmol) was added to a mixed solvent composed of toluene (100g, 20 times the mass of compound 2-a) and water (100g, 20 times the mass of compound 2-a), stirred and mixed and then added three (o-tolyl) ) phosphine (5%eq, 0.08g, 304.37g / mol, 0.27mmol) and palladium (II) acetate (1%eq, 0.01g, 224.51g / mol, 0.05mmol), warming up to reflux and stirring for 15h, the reaction ended After cooling to room temperature, filter, separate the toluene, extract the aqueous phase with toluene, combine the organic phases, wash with water, dry and concentrate under reduced pressure to obtain the crude product, which is purified by column chromatography to obtain the organic compound (4) (3.87g, Yield 77.5%), MS (EI): 915.3 (M+H) + .
Embodiment 3
[0042]
[0043] The synthetic method of organic compound (15) is as follows:
[0044]
[0045] Compound 3-a (5g, 1200.27g / mol, 4.17mmol), compound 3-b (2.1eq, 1.08g, 123.05g / mol, 8.75mmol) and sodium carbonate (4eq, 1.77g, 105.99g / mol, 16.66mmol) was added into a mixed solvent composed of toluene (100g, 20 times the mass of compound 3-a) and water (100g, 20 times the mass of compound 3-a), stirred and mixed and then added three (o-tolyl) ) phosphine (5%eq, 0.06g, 304.37g / mol, 0.21mmol) and palladium (II) acetate (1%eq, 0.01g, 224.51g / mol, 0.04mmol), warming up to reflux and stirring for 15h, the reaction ended After cooling to room temperature, filter, separate the toluene, extract the aqueous phase with toluene, combine the organic phases, wash with water, dry and concentrate under reduced pressure to obtain the crude product, which is purified by column chromatography to obtain the organic compound (15) (3.71g, Yield 74.3%), MS (EI): 1199.5 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com