Application of compound AD-35 for treating gastrointestinal dysperistalsis related diseases
A technology of gastrointestinal peristalsis and compounds, applied in the field of medicine, can solve problems such as strong side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
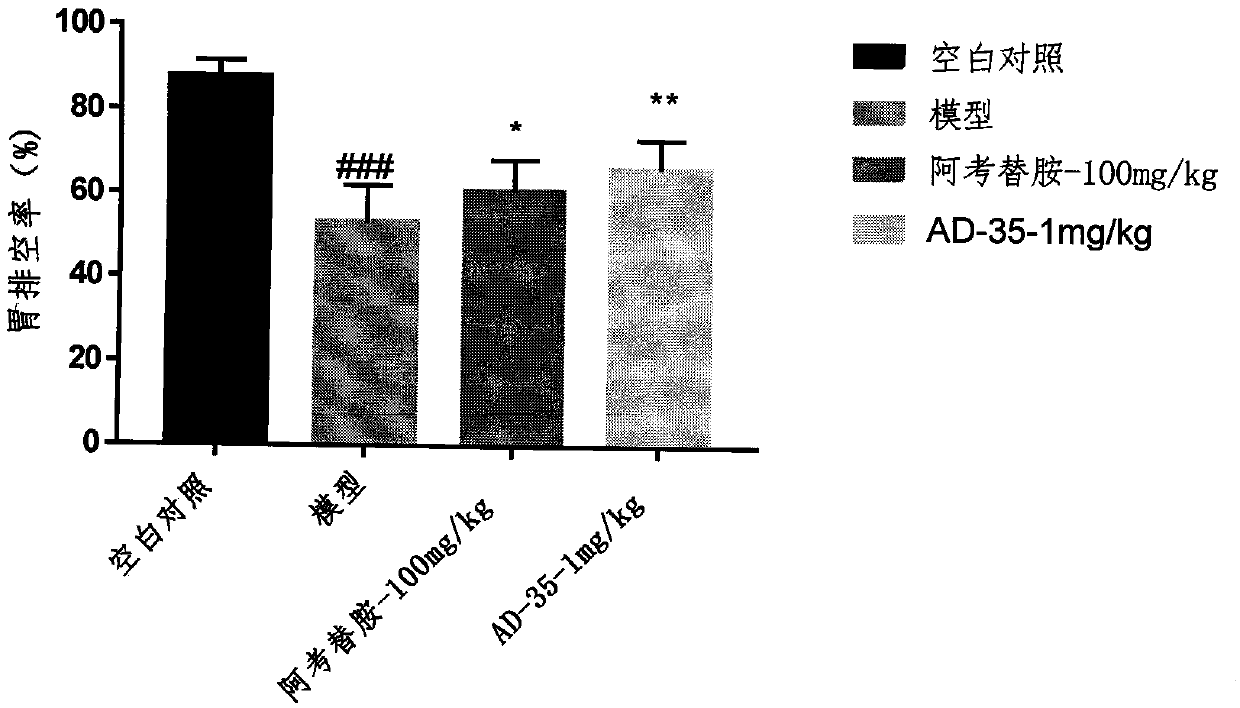
[0022] Example 1: Improvement effect of compound AD-35 on delayed gastric emptying in mice induced by clonidine
[0023] ICR mice were randomly divided into groups according to body weight, and fasted for 24 hours. After 55 minutes of administration of the drug group (the model group and the blank control group were given normal saline), the model-making agent clonidine (0.1 mg / kg) was subcutaneously injected into the test animals, and phenol red solution (0.4 ml / only) was administered by intragastric administration 5 minutes later. , and the mice were sacrificed after 30 min. Take out the stomach and place it in 10ml of 0.1M NaOH solution, cut the stomach tissue with scissors to release the stomach contents, centrifuge at 5000rpm for 10min, take 1ml of supernatant, add 0.1ml of 20% trichloroacetic acid to it, and centrifuge at 5000rpm for 20min , take 0.5ml of supernatant, add 0.2ml of 0.5M NaOH solution into it, mix well, then take the supernatant and detect the OD value at...
Embodiment 2
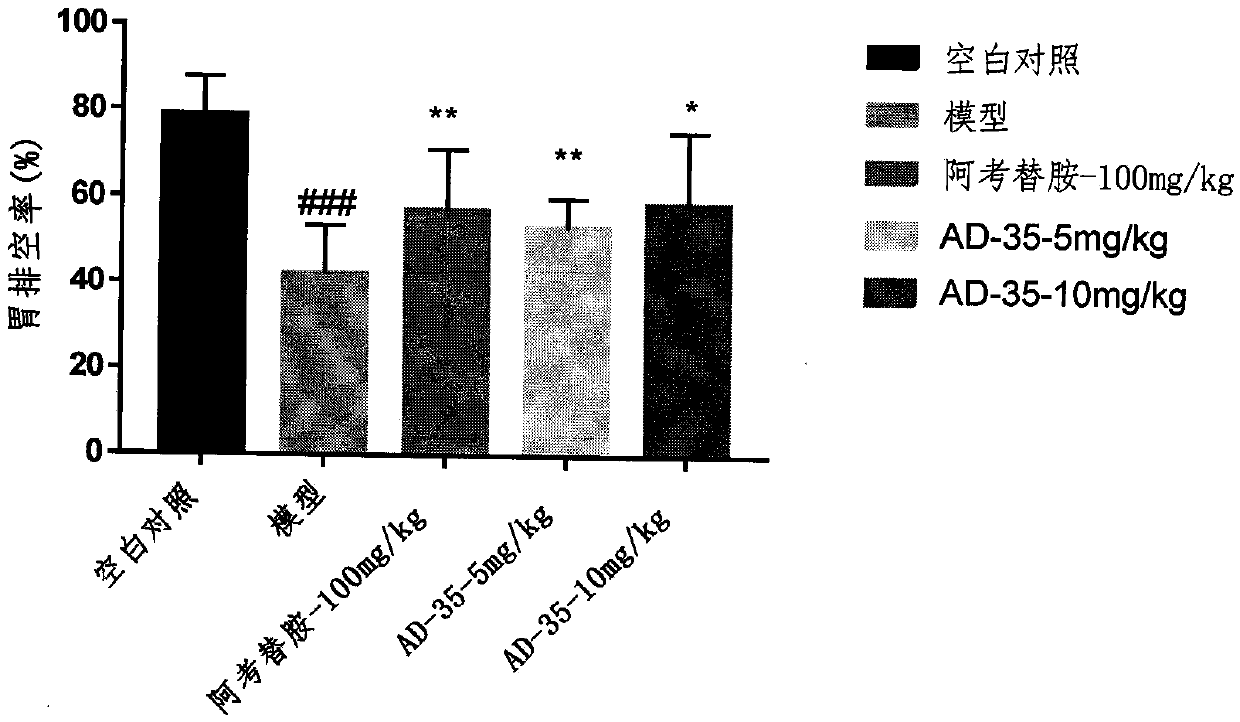
[0024] Example 2 Compound AD-35 improves the delayed gastric emptying induced by clonidine
[0025] SD rats were randomly divided into groups according to body weight, and fasted for 24 hours. After 55 minutes of drug group administration (the model group and the blank control group were given normal saline), the model-making agent clonidine (0.2 mg / kg) was subcutaneously injected into the experimental animals, and phenol red solution (1.6 ml / only) was administered by intragastric administration 5 minutes later. , and the rats were killed by cervical dislocation after 30 min. Take out the stomach and place it in 50ml of 0.1M NaOH solution, cut the stomach tissue with scissors to release the stomach contents, centrifuge at 5000rpm for 10min, take 1ml of supernatant, add 0.1ml of 20% trichloroacetic acid to it, and then centrifuge at 5000rpm for 20min , take 0.5ml of supernatant, add 0.2ml of 0.5M NaOH solution into it, mix well, take the supernatant and detect the OD value at ...
Embodiment 3
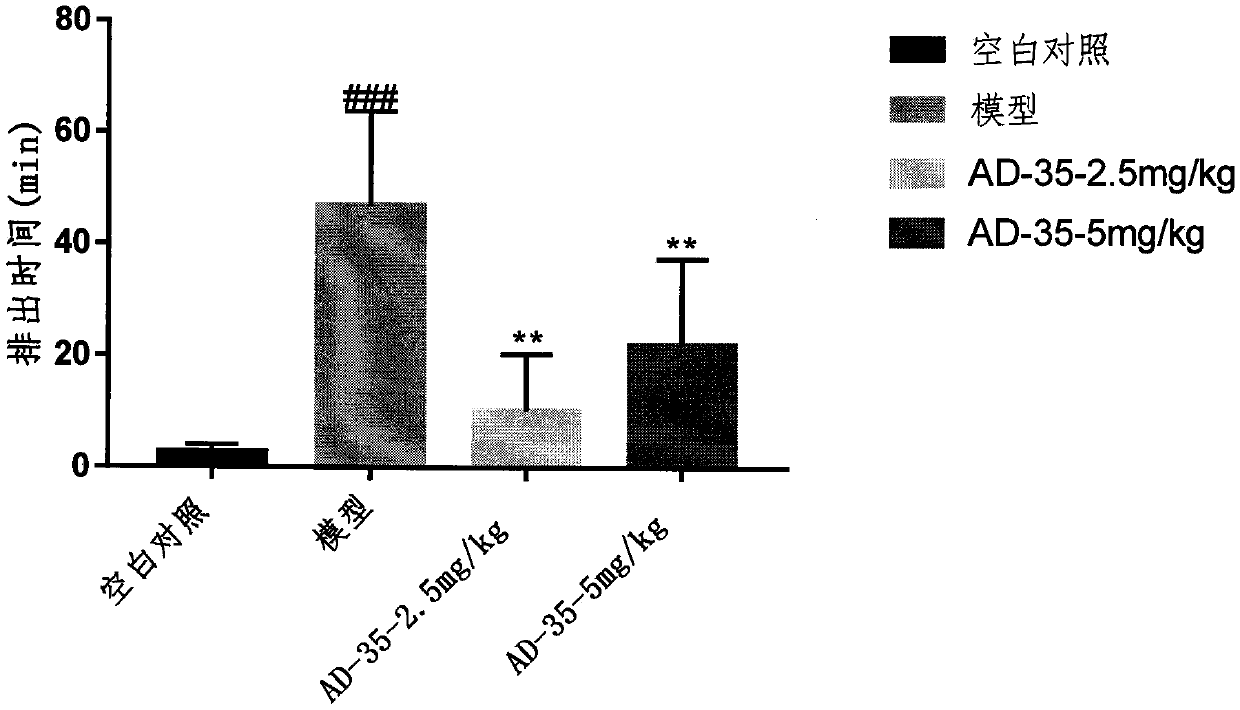
[0026] Example 3 Compound AD-35 improves the delay of loperamide-induced intestinal propulsion in mice
[0027] ICR mice were subcutaneously injected with loperamide (5 mg / kg), once a day, for 7 consecutive days to establish models. On the seventh day, a plastic ball with a diameter of 3 mm was pushed 3 cm into the rectum of the mouse, and the time for the plastic ball to be discharged from the rectum was observed and recorded. The mice were randomly grouped according to the time when the plastic ball was discharged from the rectum, and different doses of AD-35 were administered to the mice in different administration groups on the second day after grouping (denoted as day1). The mice in each administration group were continuously given different doses of AD-35 on day 1-day 6 (the model group and the normal control group were given normal saline), and then injected with loperamide (5 mg / kg) 30 minutes later; after fasting for 4 hours on day 7, Different doses of AD-35 were ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com