Preparation method of multi-transition metal nitride zinc-air battery cathode material
A technology of zinc nitride and cathode material is applied in battery electrodes, fuel cell type half cells and secondary battery type half cells, circuits, etc. Strong charge-discharge long-term cycle stability, rich pore structure, and anti-aggregation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) After dissolving 0.45g of guanidine carbonate in 30mL of deionized water, add 0.15g of Co(CH 3 COO) 2 ·6H 2 O, stirred at room temperature for 10min; subsequently, added 0.80g Zn(NO 3 ) 2 ·6H 2 O, and after stirring at room temperature for 30min, a transition metal salt solution was formed;
[0038] (2) Add 0.3 g of locust bean gum to the transition metal solution, and stir at room temperature for 3 hours to obtain a viscous liquid;
[0039](3) freeze-drying the viscous liquid obtained in step (2) at -85°C to obtain a spongy solid;
[0040] (4) Put the spongy solid in step (3) into a covered porcelain crucible and then put it into a tube furnace, then raise the temperature to 900°C at a heating rate of 5°C / min under an argon (Ar) atmosphere Argon (Ar), and kept at this temperature for 1.5h; to be naturally cooled to room temperature to obtain a black powder. Named Zn / CoN-NC.
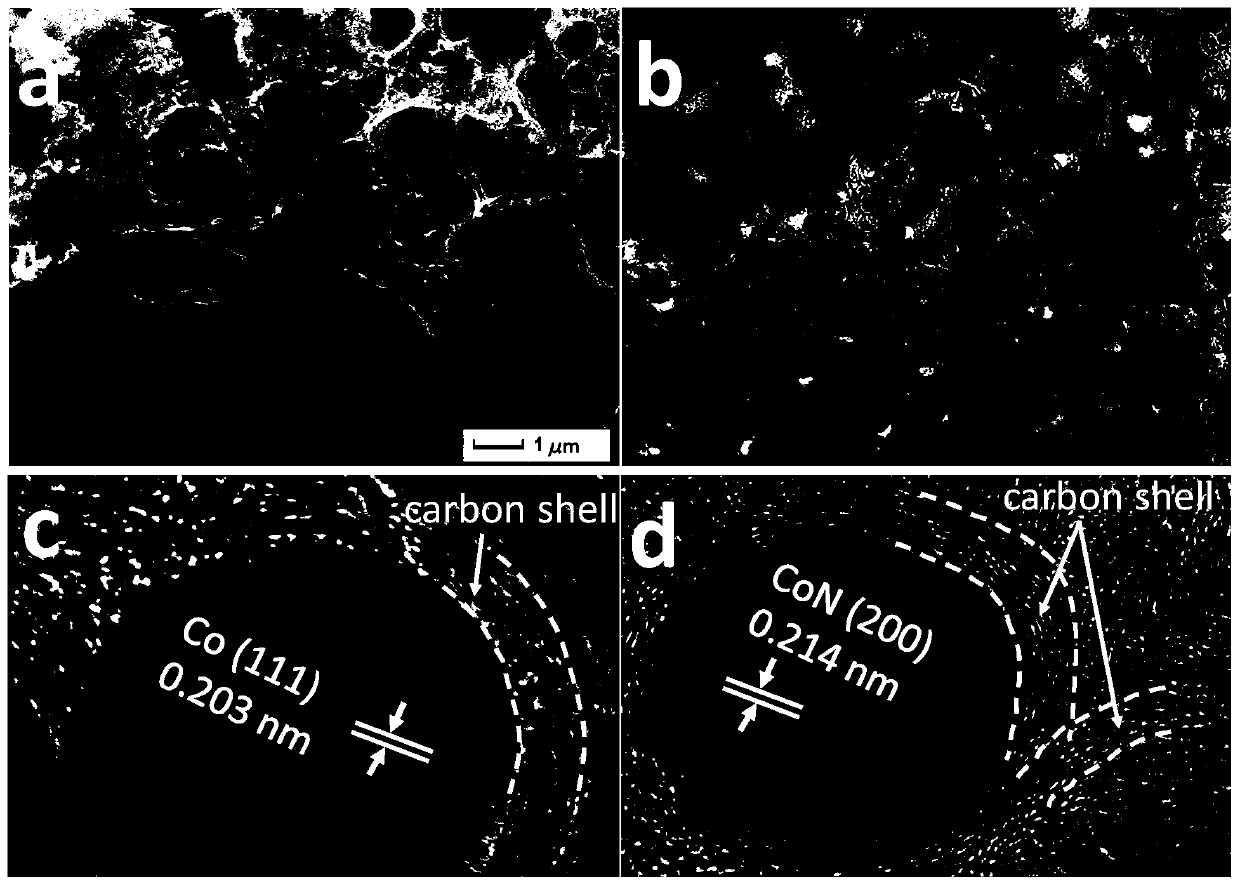
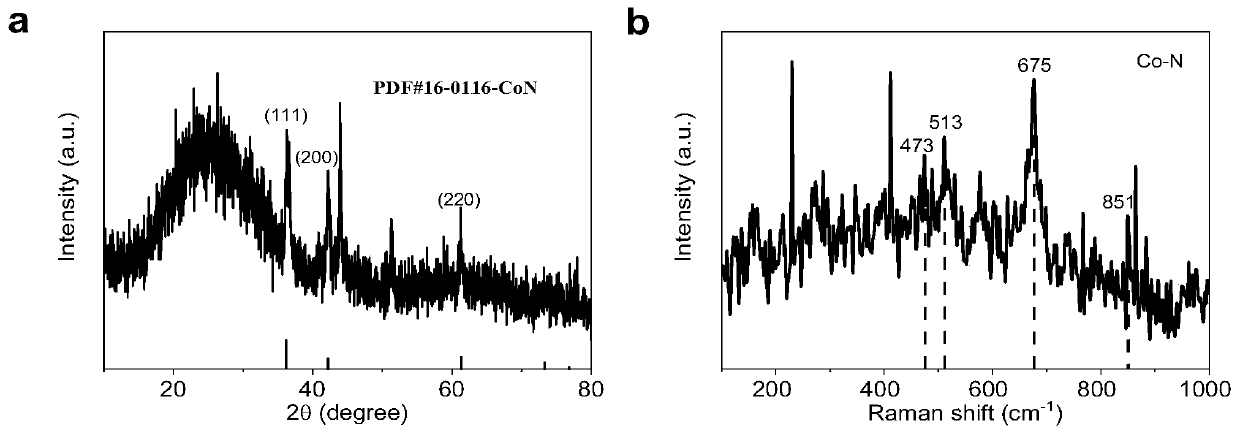
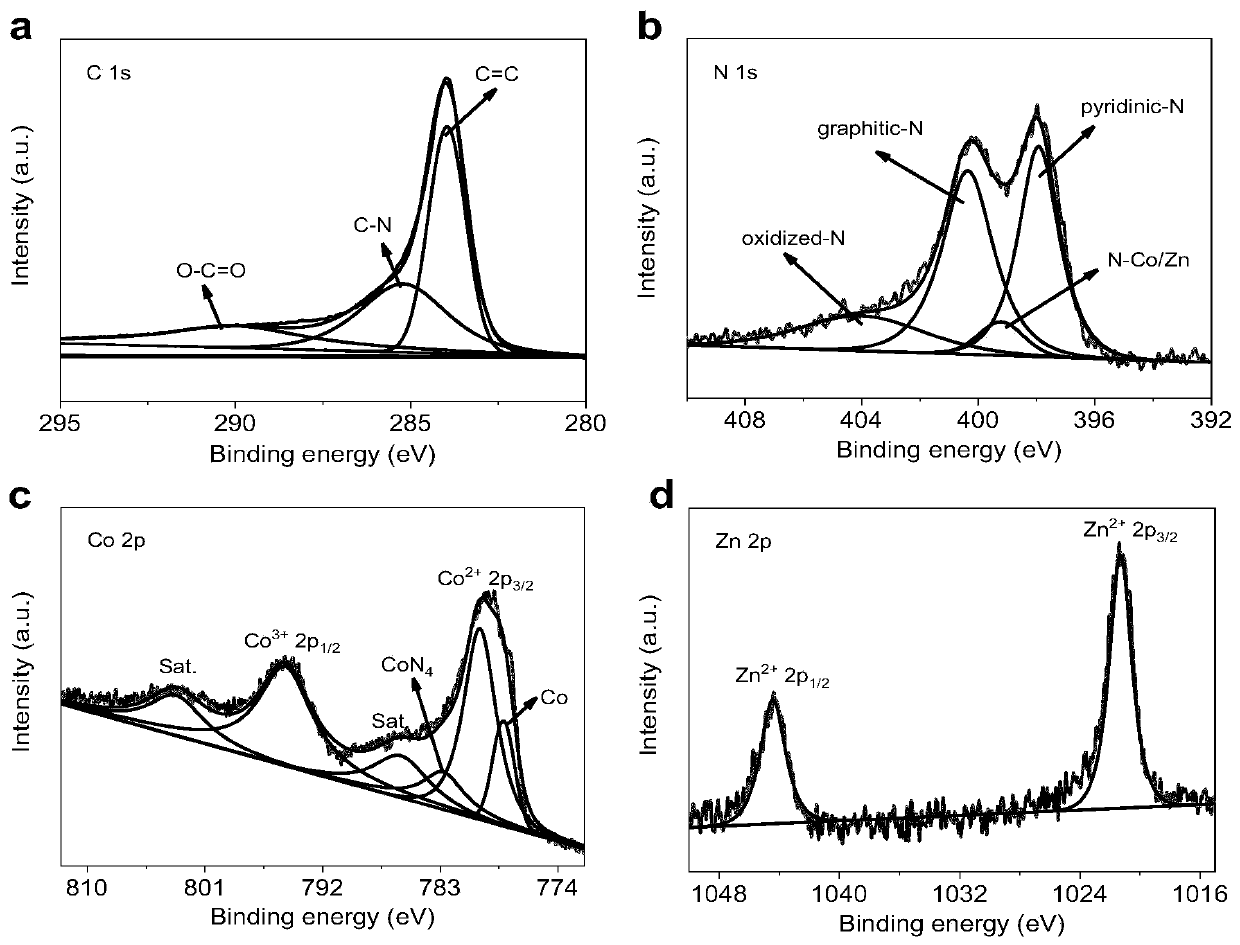
[0041] The final electrocatalysts obtained above were characterized by X-ray diffr...
Embodiment 2
[0043] (1) After dissolving 0.45g of guanidine carbonate in 30mL of deionized water, add 0.12g of MnCl 2 4H 2 O, stirred at room temperature for 10min; subsequently, added 0.80g Zn(NO 3 ) 2 ·6H 2 O, and after stirring at room temperature for 30min, a transition metal salt solution was formed;
[0044] (2) Add 0.3 g of locust bean gum to the transition metal solution, and stir at room temperature for 3 hours to obtain a viscous liquid;
[0045] (3) freeze-drying the viscous liquid obtained in step (2) at -85°C to obtain a spongy solid;
[0046] (4) Put the spongy solid in step (3) into a covered porcelain crucible and then put it into a tube furnace, then raise the temperature to 900°C at a heating rate of 5°C / min under an argon (Ar) atmosphere Argon (Ar), and kept at this temperature for 1.5h; to be naturally cooled to room temperature to obtain a black powder. Named Zn / MnNC.
[0047] The final electrocatalysts obtained above were characterized by X-ray diffraction (XRD...
Embodiment 3
[0049] (1) After dissolving 0.45g of guanidine carbonate in 30mL of deionized water, add 0.80g of Zn(NO 3 ) 2 ·6H 2 O, and stirred at room temperature for 10 min; subsequently, 0.034 g of CuCl was added to the above aqueous solution 2 2H 2 O forms a transition metal salt solution;
[0050] (2) Add 0.3 g of locust bean gum to the transition metal solution, and stir at room temperature for 3 hours to obtain a viscous liquid;
[0051] (3) freeze-drying the viscous liquid obtained in step (2) at -85°C to obtain a spongy solid;
[0052] (4) Put the spongy solid in step (3) into a covered porcelain crucible and then put it into a tube furnace, then raise the temperature to 900°C at a heating rate of 5°C / min under an argon (Ar) atmosphere Argon (Ar), and kept at this temperature for 1.5h; to be naturally cooled to room temperature to obtain a black powder. Named Zn / CuNC.
[0053] The final electrocatalysts obtained above were characterized by X-ray diffraction (XRD), scanning ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com