Boron-doped carbon material and its preparation method and use
A technology of boron doping and carbon materials, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of difficult control of process conditions in the preparation process, unfavorable catalyst industrialization, complex preparation methods, etc., and achieve excellent stability and methanol resistance performance, good ORR catalytic activity, and high degree of graphitization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
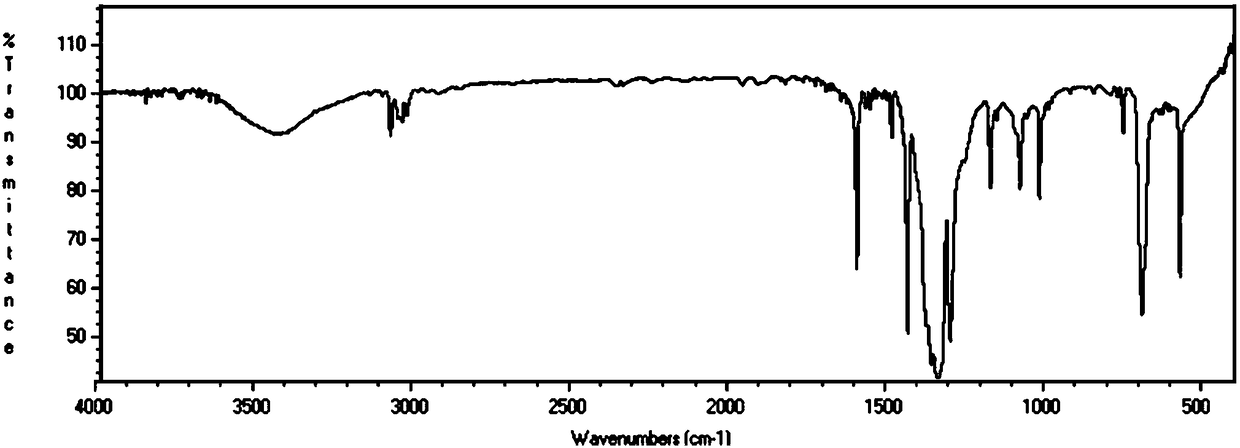
[0069] (1) Synthesis of PTPB: Dissolve 62.3g (0.2mol) triphenyltriboroxane (TPB) in 2000mL nitrobenzene, then add 91.3g (1.2mol) dimethoxymethane (molecular weight 76) and 289.7g (1.2mol) of anhydrous aluminum trichloride, stirred at 45°C for 5h, then raised to 80°C and stirred for 19h; after the reaction was completed, poured into 10L of methanol for settling, filtered, washed with water (1000mL×2), and dried for later use; The polymer was characterized by infrared spectroscopy, as figure 1 shown;
[0070] (2) 2.5g PTPB in N 2 Heat treatment at 900°C for 2 hours in the atmosphere to obtain 1.5 g of boron-doped carbon-nonmetal oxygen reduction catalyst, which is designated as B-C-900.
[0071] Catalyst performance test method (the following each embodiment obtains catalyst and the commercial Pt / C (JohnsonMatthey) that is used for comparison all tests with this method)
[0072] (1) Weigh 2mg of the catalyst, add it to 1mL Nafion-ethanol (1.5 / 98.5vol / vol) solution, ultrasonic...
Embodiment 2
[0077] (1) Synthesis of PTPB: Dissolve 62.3g (0.2mol) triphenylboroxane (TPB) in 2000mL dichloroethane, then add 91.3g (1.2mol) dimethoxymethane and 289.7g ( 1.2mol) of anhydrous aluminum trichloride, stirred at 45°C for 5h, then raised to 80°C and stirred for 19h; after the reaction was completed, poured into 10L methanol for sedimentation, filtered, washed with water (1000mL×2), and dried for later use; the obtained polymer was Infrared spectroscopy was characterized as figure 1 shown;
[0078] (2) 2.5g PTPB in N 2 Heat treatment at 800°C for 2 hours in the atmosphere to obtain 1.2 g of boron-doped carbon-nonmetal oxygen reduction catalyst, which is designated as B-C-800.
Embodiment 3
[0080] (1) Synthesis of PTPB: Dissolve 62.3g (0.2mol) triphenyltriboroxane (TPB) in 2000mL nitrobenzene, then add 88.9g (1.2mol) diethyl ether (molecular weight 74), 45.6 g (0.6mol) dimethoxymethane and 194.6g (1.2mol) anhydrous ferric trichloride, stirred at 45°C for 5h, then raised to 80°C and stirred for 19h; after the reaction was completed, pour it into 10L methanol for sedimentation, filter, Washed with water (1000mL × 2), dried for subsequent use; The resulting polymer was characterized by infrared spectroscopy, as figure 1 shown;
[0081] (2) 2.5g PTPB in N 2 Heat treatment at 1000°C for 2 hours in the atmosphere to obtain 1.1 g of boron-doped carbon-nonmetal oxygen reduction catalyst, which is designated as B-C-1000.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com