Immobilized chiral catalyst, preparation method thereof, and synthesis method of chiral compounds
A chiral catalyst and solid-phase technology, which is applied in the preparation of organic compounds, physical/chemical process catalysts, ruthenium organic compounds, etc., can solve the problems of low stability, difficult recovery and reuse, and low conversion number. Achieve the effect of reducing synthesis cost, solving the risk of metal residue, and ensuring the catalytic conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
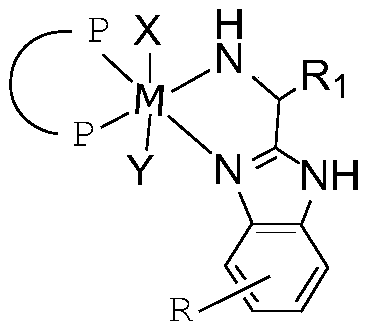
[0052] The preparation method of the above-mentioned solid-phased chiral catalyst comprises the steps:
[0053] step one:
[0054]
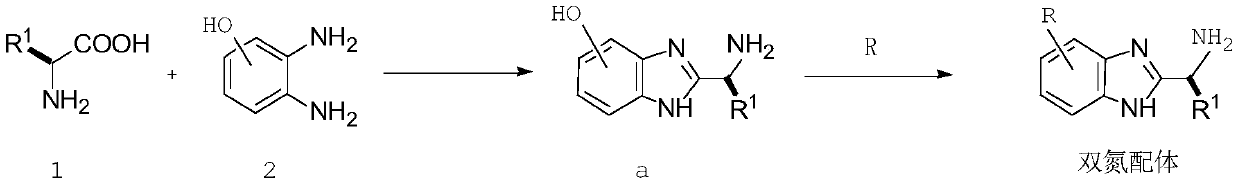
[0055] 1) react raw material 1 and raw material 2 to obtain intermediate a;
[0056] 2) Substituting the intermediate a with the high molecular polymer R to obtain a dinitrogen ligand containing the high molecular polymer R;
[0057] Step two:
[0058] The transition metal compound, the bisphosphine ligand and the dinitrogen ligand described in step 1 are mixed in an organic solvent to perform a coordination reaction.
[0059] In one specific embodiment, in 2) of step 1, the temperature of the reaction is 60-90° C., and the time is 5-10 hours.
[0060] In one specific embodiment, in step 2, the conditions of the coordination reaction include: the reaction temperature is 20°C-120°C, and the time is 0.5h-20h. Preferably, the reaction temperature is 90°C-110°C, and the time is 0.5h-2h.
[0061] In one specific embodiment, in step 2, the molar ...
Embodiment 1
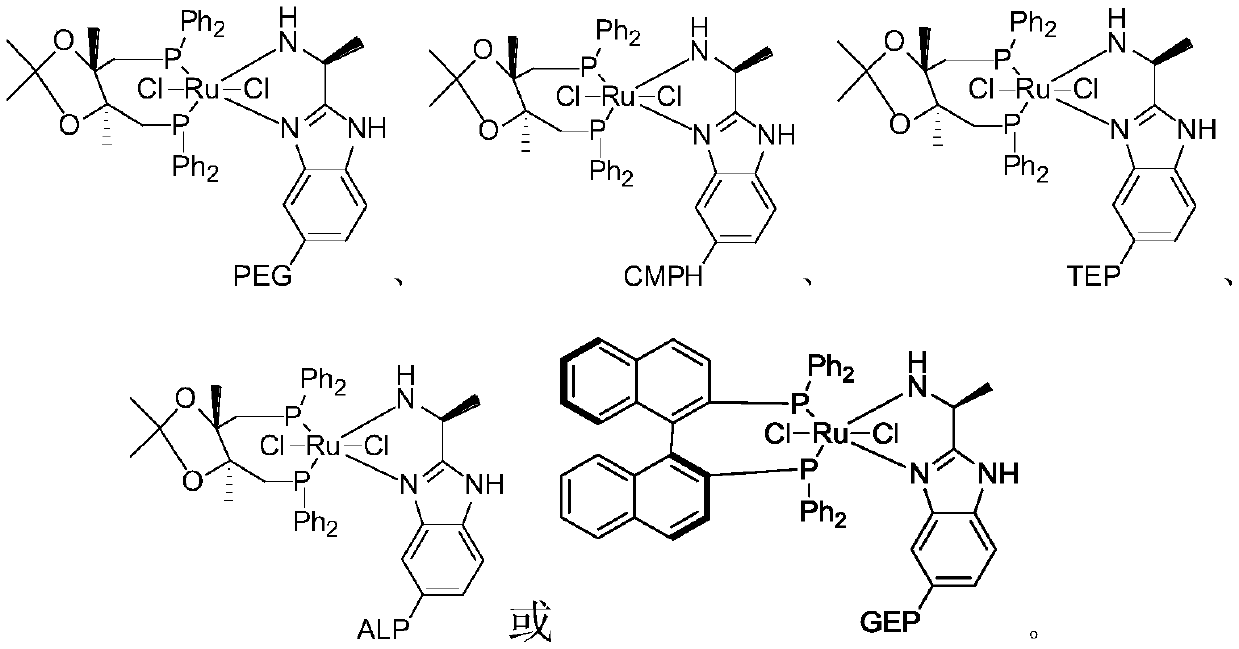
[0066] This embodiment is a kind of solid phase chiral catalyst (S)-1, its structure is as follows:
[0067]
[0068] Its preparation method steps are as follows:
[0069] Step 1: Synthesis of dinitrogen ligand:
[0070]
[0071] 1) Using dichloromethane as a solvent and EDC.HCl as a condensation agent, mix 1.1 mol of raw material 1 and 1.2 mol of raw material 2 at room temperature for condensation reaction for 5 hours. After the reaction is completed, add water to the reaction solution, separate and collect the organic phase , dry, and remove the solvent to obtain intermediate a;
[0072] 2) Mix 1.1 mol of intermediate a with 1.2 mol of polyethylene glycol (PEG), use DMF as a solvent, react at 80°C for 8 hours, and then filter with suction to collect the solid to obtain a dinitrogen complex containing polymer R. body;
[0073] Step 2: Synthesis of (S)-1
[0074]
[0075] Under the protection of argon, the [RuCl 2 (C 6 h 6 )] 2 8.2 mg and 20 mg of (S)-Diop (p...
Embodiment 2
[0077] This example is a solid-phase chiral catalyst (S)-2, the preparation method of which is similar to Example 1, except that the high molecular polymer used is cellulose. Its preparation method steps are as follows:
[0078] Step 1: Synthesis of dinitrogen ligand:
[0079]
[0080] 1) The preparation of intermediate a is the same as in Example 1;
[0081] 2) Mix 1.1 mol of intermediate a with 1.2 mol of hydroxypropylmethylcellulose (HPMC), use DMF as a solvent, react at 80°C for 8 hours, and then filter with suction to collect the solid, that is, the compound containing polymer R is obtained. Dinitrogen ligand;
[0082] Step 2: Synthesis of (S)-2
[0083]
[0084] Under the protection of argon, the [RuCl 2 (C 6 h 6 )] 2 8.2 mg and 20 mg of (S)-Diop (purchased from Aladdin) were suspended in 2 mL of DMF degassed with argon, and stirred at 100°C for 1 hour; the solvent was removed in vacuo at 50°C to obtain a brown solid; then, bis Nitrogen ligand 7.2 mg and 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com