A kind of preparation method of targeted drug delivery nano-delivery system
A technology of targeted drug delivery and delivery system, which is applied in the field of preparation of targeted drug delivery nano-delivery system, can solve problems such as insoluble in water and difficult to use, and achieve increased intracellular throughput, small solvent consumption, and reduced Toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of a targeted drug delivery nano-delivery system:
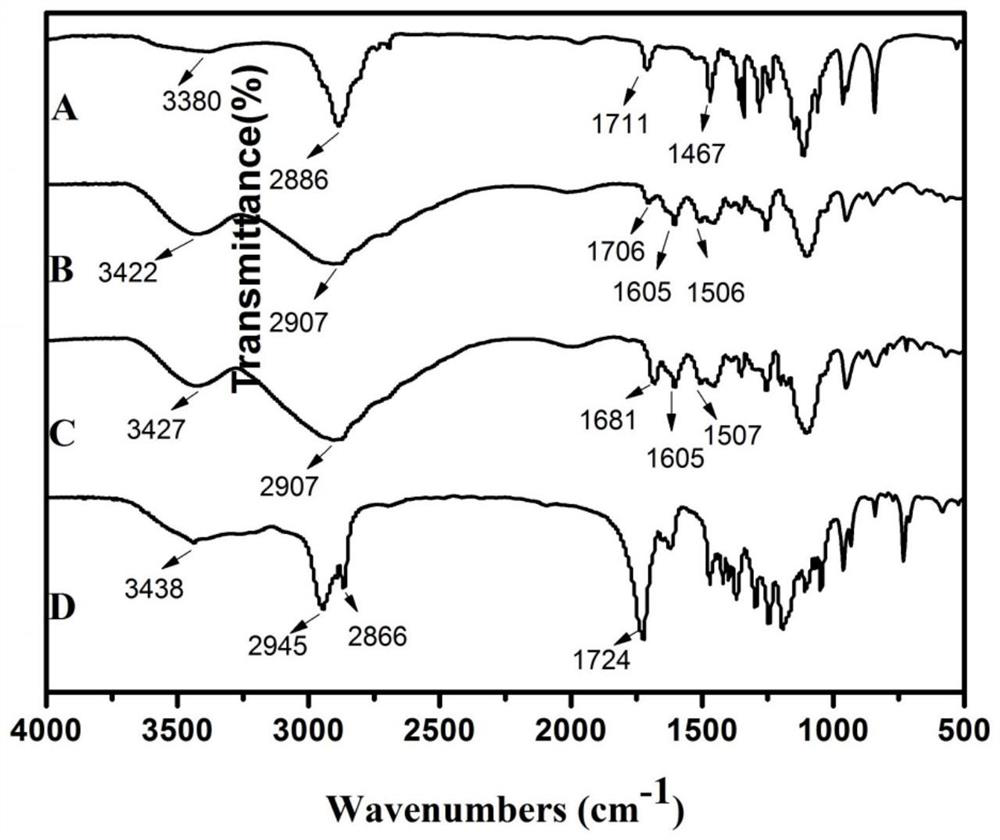
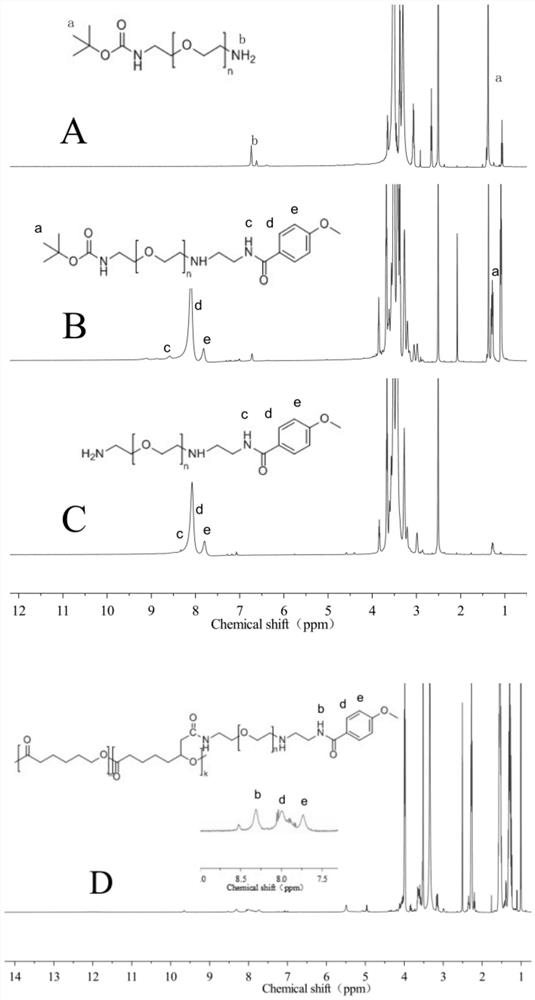
[0039] (1) p-methoxybenzoyl chloride (341mg, 2mmol), 2-bromoethylamine hydrobromic acid (410mg, 2mmol), N,N-diisopropylethylamine (DIPEA) 180μL was dissolved in 5ml acetonitrile, 40°C for 12h, adding NH 2 -PEG-NH-Boc(M w = 2000) (400 mg, 2 mmol), stirred at room temperature for 24 hours; add 30 mL of ether to the reaction solution, centrifuge at 10,000 RCF for 10 minutes, store the tube at -20 °C for 2 hours, and centrifuge at 8000 RPM and -4 °C for 5 minutes to form dense Precipitate, remove waste ether, add new ether, vortex and centrifuge at -4°C for 5 minutes at 8000 RPM to form a dense precipitate to obtain AEAA-PEG-NH-Boc with a yield of 60.2%.
[0040] (2) Re-suspend the precipitated AEAA-PEG-NH-Boc 240.8 mg in 4.5 mL of trifluoroacetic acid (TFA):dichloromethane (DCM) volume ratio 1:2, stir the solution for 2 hours, and the solvent is under the rotating kettle Evaporate three times, add DCM to resu...
Embodiment 2
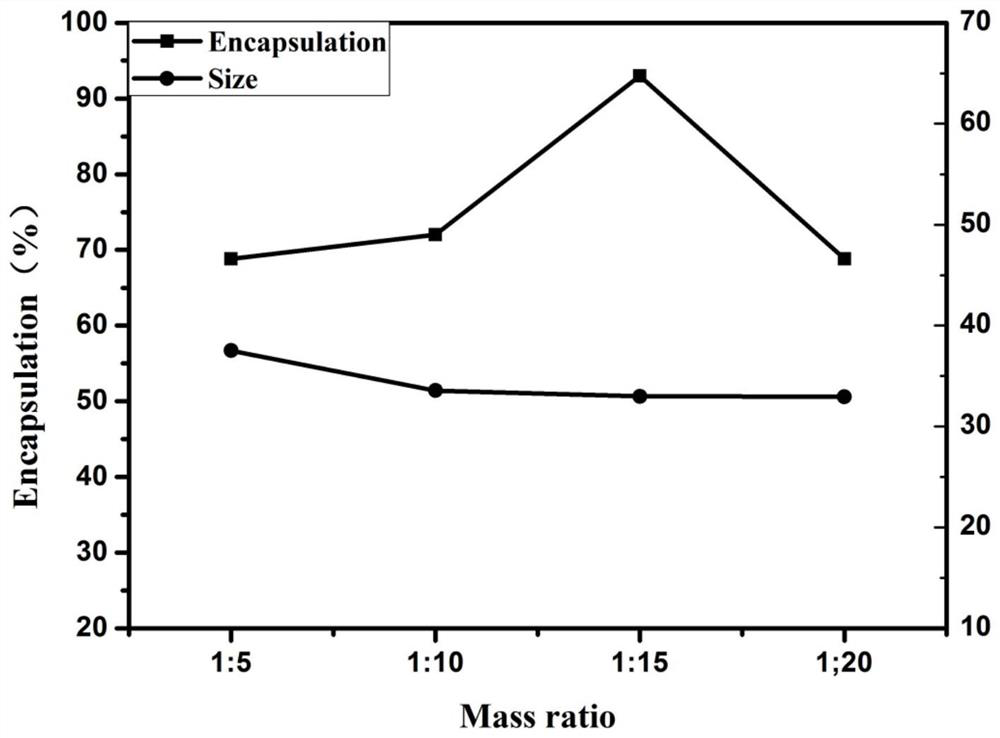
[0047] The specific operation steps of this example are basically the same as those of Example 1, except that the mass ratio of the polymer carrier AEAA-PEG-PCL to the hydrophobic drug IPI-549 in step (4) of this example is 5:1.
[0048] The encapsulation efficiency and particle size of the nanoparticles prepared in this example were 68.8% and 37.52 nm.
Embodiment 3
[0050] The specific operation steps of this example are basically the same as those of Example 1, except that the mass ratio of the polymer carrier AEAA-PEG-PCL to the hydrophobic drug IPI-549 in step (4) of this example is 10:1.
[0051] The encapsulation efficiency and particle size of the nanoparticles prepared in this example were 72% and 33.56 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com