Application of bergenin in preparation of medicine for treating Klebsiella pneumoniae infection in vivo
A technology of Klebsiella and petrocyanin, applied in the direction of antibacterial drugs, drug combinations, antipyretics, etc., to achieve the effects of improving lung damage, reducing use, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
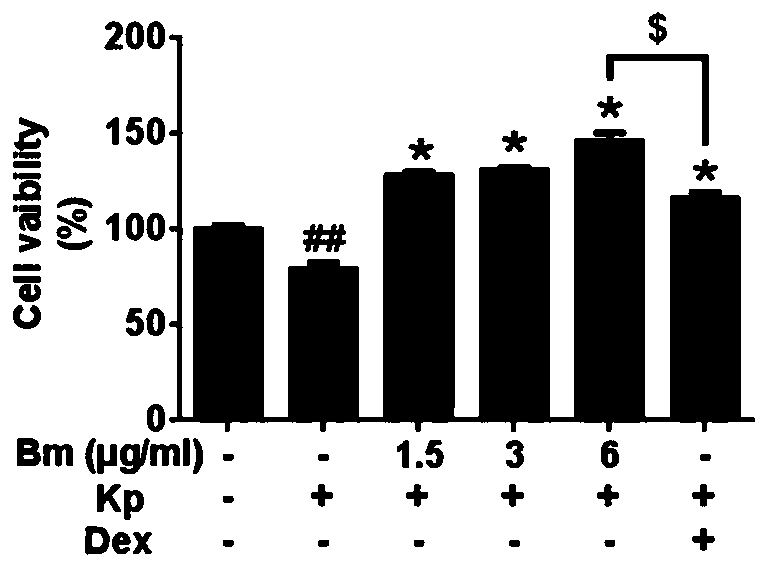
[0034] Effect of petracenin on cell viability
[0035] Experimental method:
[0036] The experiment was divided into 6 groups, namely the blank control group, the Klebsiella pneumoniae group, the low-dose begenin group, the middle-dose begenin group, the high-dose begenin group, and the dexamethasone group.
[0037] It is handled as follows:
[0038] RAW 264.7 cells were seeded into 96-well plates (2×10 6 cells / well) for 24 hours, then add low, medium and high doses of petracenin (1.5, 3, 6 μg / ml), and treat for 1 hour; and add dexamethasone (Dexamethasone, Dex) as a positive control (100 μg / ml), treatment time 1 hour; then the cells were exposed to the Klebsiella pneumoniae environment for 24 hours; the blank control group did not carry out any treatment; the Klebsiella pneumoniae group only carried out Klebsiella pneumoniae treatment , without adding other drugs.
[0039] Subsequently, the 96-well plate was washed twice with PBS, MTT (5 mg / ml) was added to the cells, and...
Embodiment 2
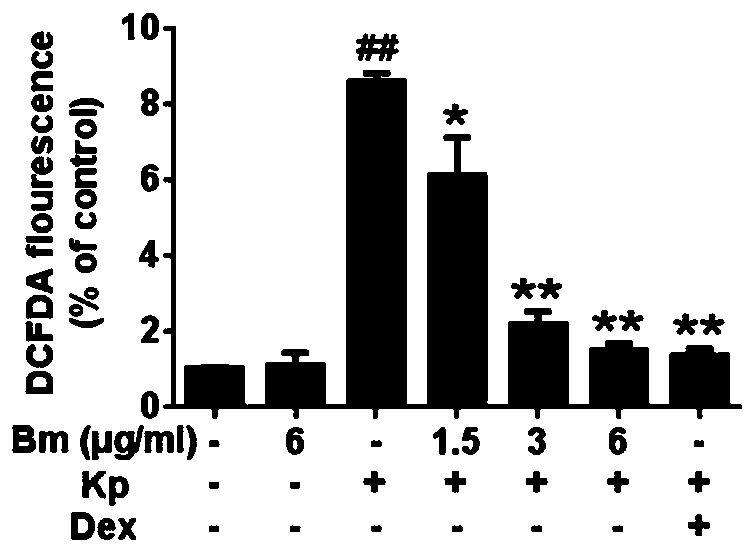
[0046] Effects of petracenin on antioxidant activity of cells
[0047] Experimental method:
[0048] The experiment was divided into 6 groups, namely: blank control group, Klebsiella pneumoniae group, low-dose petracenin group, middle-dose petracenin group, high-dose petracenin group, and dexamethasone group.
[0049] It is handled as follows:
[0050] 1. Inoculate RAW 264.7 cells into a 96-well plate (2×10 4 cells / well) for 24 hours, then add low, medium and high doses of petracenin (1.5, 3, 6 μg / ml) for 1 hour; and add dexamethasone (Dex) as a positive control (100 μg / ml), the treatment time was 1 hour; then the cells were exposed to the Klebsiella pneumoniae environment for 24 hours; the blank control group was not treated, and the Klebsiella pneumoniae group was only treated with Klebsiella pneumoniae without adding other medicines.
[0051] Afterwards, the cells were washed twice with ice-cold PBS, and stained with DCFH-DA for 20 min.
[0052] 2. Measure ROS generat...
Embodiment 3
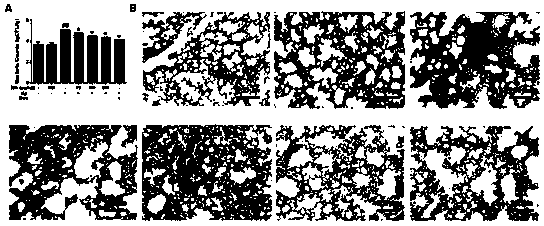
[0060] Anti-inflammatory effect of petrinin on pneumonia mice infected by Klebsiella pneumoniae
[0061] The experiment was divided into 6 groups, namely: blank control group, Klebsiella pneumoniae group, low-dose petracenin group, middle-dose petracenin group, high-dose petracenin group, and dexamethasone group.
[0062] 1. Establishment and challenge of animal model
[0063] BALB / c male mice were divided into 6 groups: blank control group, Klebsiella pneumoniae group, dexamethasone group (100 μg / ml), petracenin low, middle and high dose groups (75, 150, 300 μg / kg), 15 in each group. Dexamethasone, low, medium and high doses of petracenin were injected subcutaneously into mice. One hour later, in addition to the blank control group, 10% chloral hydrate was injected intraperitoneally for anesthesia, and 30 μl of Klebsiella suspension (2×10 7 CFU), mice were dislocated 48 hours after infection. The blank control group did not do any operation, and the Klebsiella pneumoniae...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com