Tetrahydropyridinopyrimidine compound and preparation method and application thereof
A technology for tetrahydropyridine and pyrimidine, which is applied in the field of compounds and their preparation, can solve problems such as limited synthesis methods, and achieve the effects of high atom economy, green raw materials, and cheap and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
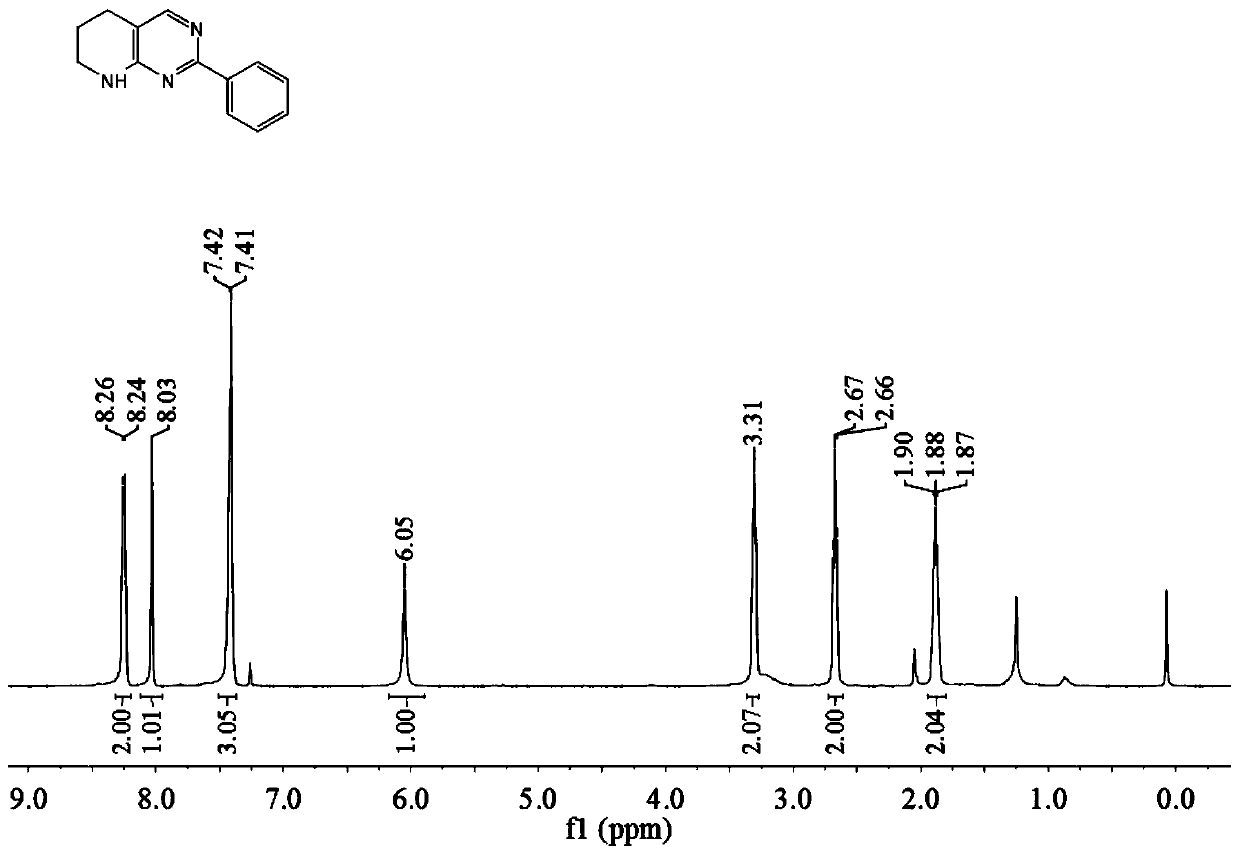
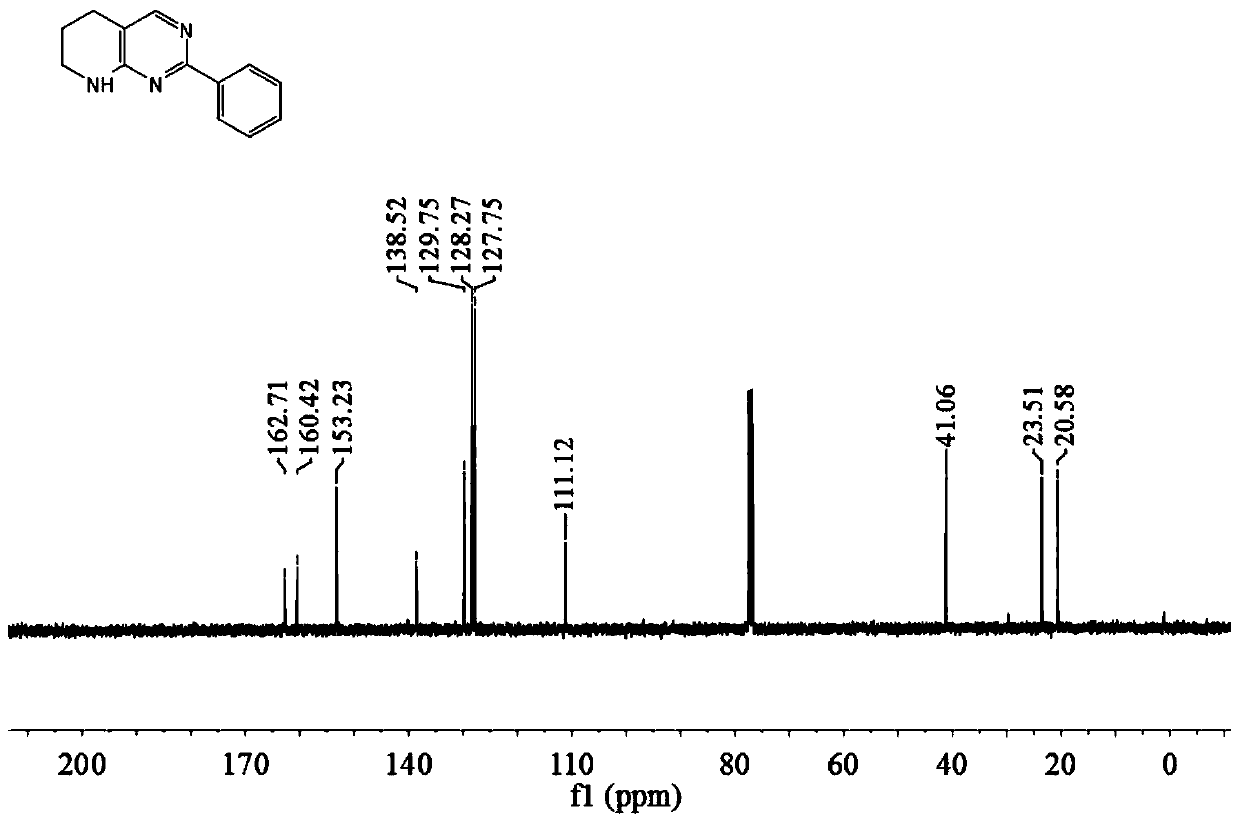
[0041] The tetrahydropyridopyrimidine compound described in this example is 2-phenyl-tetrahydropyridopyrimidine (compound shown in formula 3a), and the preparation method includes the following steps: in the reactor, add 1.0 mmol o-aminopyridinemethanol and 0.5 mmol of benzamidine, add 1% triruthenium dodecacarbonyl of the amount of ortho-aminopyridinemethanol substance, then add cesium carbonate of 50% of the amount of ortho-aminopyridinemethanol substance, add 1.5ml of methanol and 1.5ml of t- Pentanol, under nitrogen atmosphere, stirred and reacted at 70°C for 24 hours, cooled to room temperature after the reaction, diluted the reaction solution, filtered, and evaporated under reduced pressure to remove the solvent to obtain the crude product, which was purified by column chromatography The compound represented by formula 3a (yield 81%), the product was in the form of yellow oil.
[0042]
[0043] Compound hydrogen spectrogram and carbon spectrogram shown in formula 3a a...
Embodiment 2
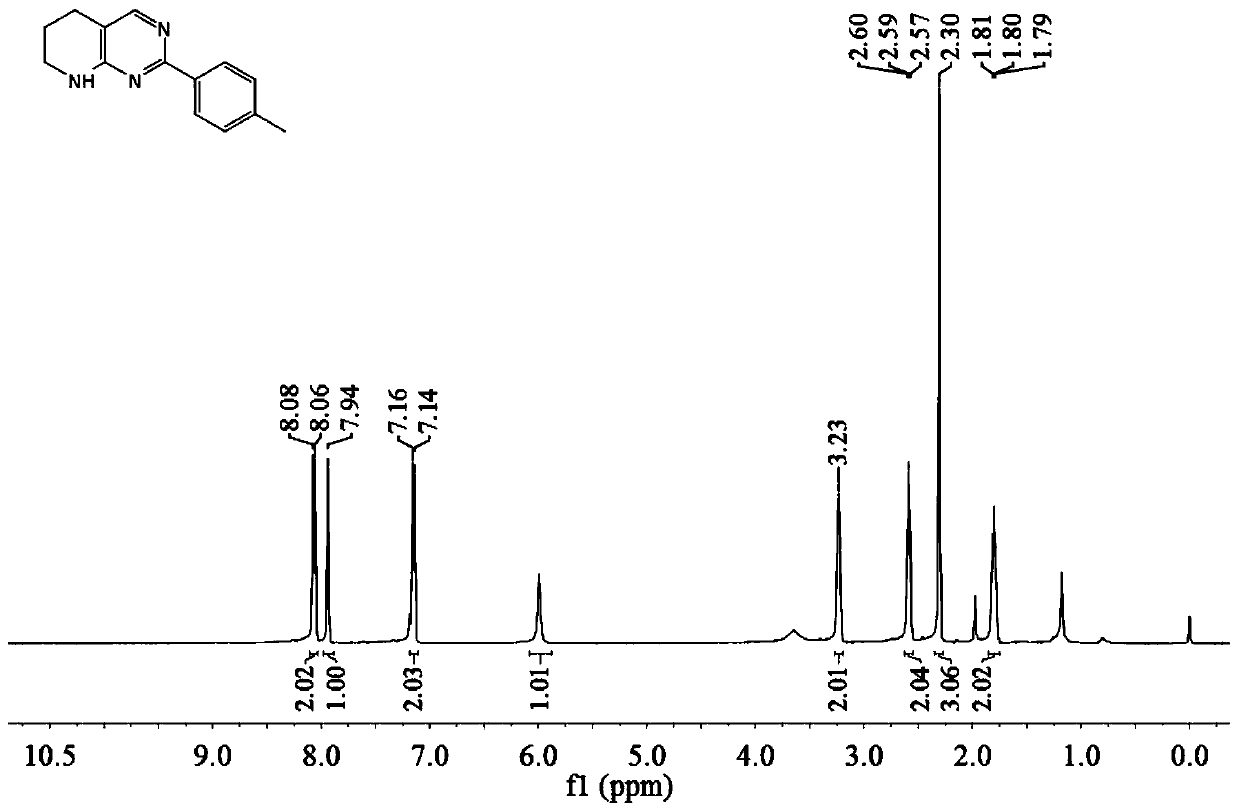
[0051] The tetrahydropyridopyrimidine compound described in this example is 2-(4-methyl-phenyl)-tetrahydropyridopyrimidine (compound shown in formula 3b), and the preparation method comprises the following steps: in the reactor , add 0.5 mmol o-aminopyridinemethanol and 1.0 mmol p-toluidine, add 3% cyclopentadienyl bis(triphenylphosphine) ruthenium(II) chloride to the amount of o-aminopyridinemethanol , then add 100% potassium tert-butoxide in the amount of o-aminopyridinemethanol, add 1.5ml isopropanol and 1.5ml tert-amyl alcohol, stir and react at 150°C for 8 hours, cool to room temperature after the reaction, and dilute the reaction solution , filtered, and the solvent was removed by rotary evaporation under reduced pressure to obtain the crude product, which was purified by column chromatography to obtain the compound shown in formula 3b (80% yield), which was in the form of yellow oil.
[0052]
[0053] The hydrogen spectrogram and the carbon spectrogram of the compoun...
Embodiment 3
[0061] The tetrahydropyridopyrimidine compound described in this example is 2-(4-chloro-phenyl)-tetrahydropyridopyrimidine (compound shown in formula 3c), and the preparation method includes the following steps: in the reactor, add 1.0 Millimoles of ortho-aminopyridinemethanol and 1.0 mmoles of 4-chloro-benzamidine, 3% dichloro(pentamethylcyclopentadienyl)iridium(III) dimer was added to the amount of ortho-aminopyridinemethanol substance (Catalyst, Cat.), add the potassium hydroxide of 50% of the amount of o-aminopyridinemethanol again, add 1.5ml ethanol and 1.5ml tert-amyl alcohol, under nitrogen atmosphere, stir reaction at 120 ℃ for 12 hours, react After cooling to room temperature, the reaction solution was diluted, filtered, and the solvent was removed by rotary evaporation under reduced pressure to obtain the crude product, which was purified by column chromatography to obtain the compound represented by formula 3c (yield 78%).
[0062]
[0063] The hydrogen spectrogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com