Hyaluronic acid detection kit and detection method
A detection kit, a technology for hyaluronic acid, applied in the field of medical analysis, can solve the problems of measurement accuracy, poor sensitivity and precision, not wide enough linear range, unfavorable wide application, etc., and achieves simple operation, fast inspection speed, and convenient separation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 kit of the present invention
[0035] 1. Preparation of HA derivatives
[0036] 1.1 Washing before BSA coupling: Add 2 times the volume of PBS buffer to the coupling container, then accurately weigh 3 mg of BSA, shake for 5 minutes, mix well, filter and discard the supernatant, and the first washing is over; repeat washing 5 times ;
[0037] 1.2 BSA surface activation: transfer the washed BSA into a small container, discard the supernatant and add 1.65 times the volume of EDC solution, then add 1.65 times the volume of NHS solution, shake and mix well, shake and react on a micro shaker at room temperature for 2 hours ;
[0038] 1.3 BSA surface activation: After the reaction, filter and discard the supernatant, transfer the activated BSA to a large container, add 33 times the volume of BSA in acetate buffer, shake for 5 minutes, mix well, filter and discard the supernatant solution, the end of one wash; repeat the wash 2 times;
[0039...
Embodiment 2
[0057] Embodiment 2 The using method of kit of the present invention
[0058] 1. Preparation before experiment
[0059] 1.1 Take the kit of Example 1 out of the refrigerated environment, and place it at room temperature (18-25°C) for 20-30 minutes to balance;
[0060] 1.2 Take a pack of lotion and dissolve it in 500ml purified water for later use;
[0061] 1.3 Adjust the temperature of the constant temperature water bath to 37°C.
[0062] 2. Experimental operation
[0063] 2.1 Add calibrator (for calibration) or sample into the reaction container (hereinafter referred to as "well") respectively, and the sample volume is 100 μl / well;
[0064] 2.2 Add 20 μl of magnetic particle suspension to each well;
[0065] 2.3 Add 20 μl of HA-binding protein solution to each well;
[0066] 2.4 Add 20 μl of enzyme conjugate to each well;
[0067] 2.5 After mixing, incubate at 37°C for 34 minutes;
[0068] 2.6 Wash with lotion 5 times;
[0069] 2.7 Add 50 μl each of luminescent substr...
Embodiment 3
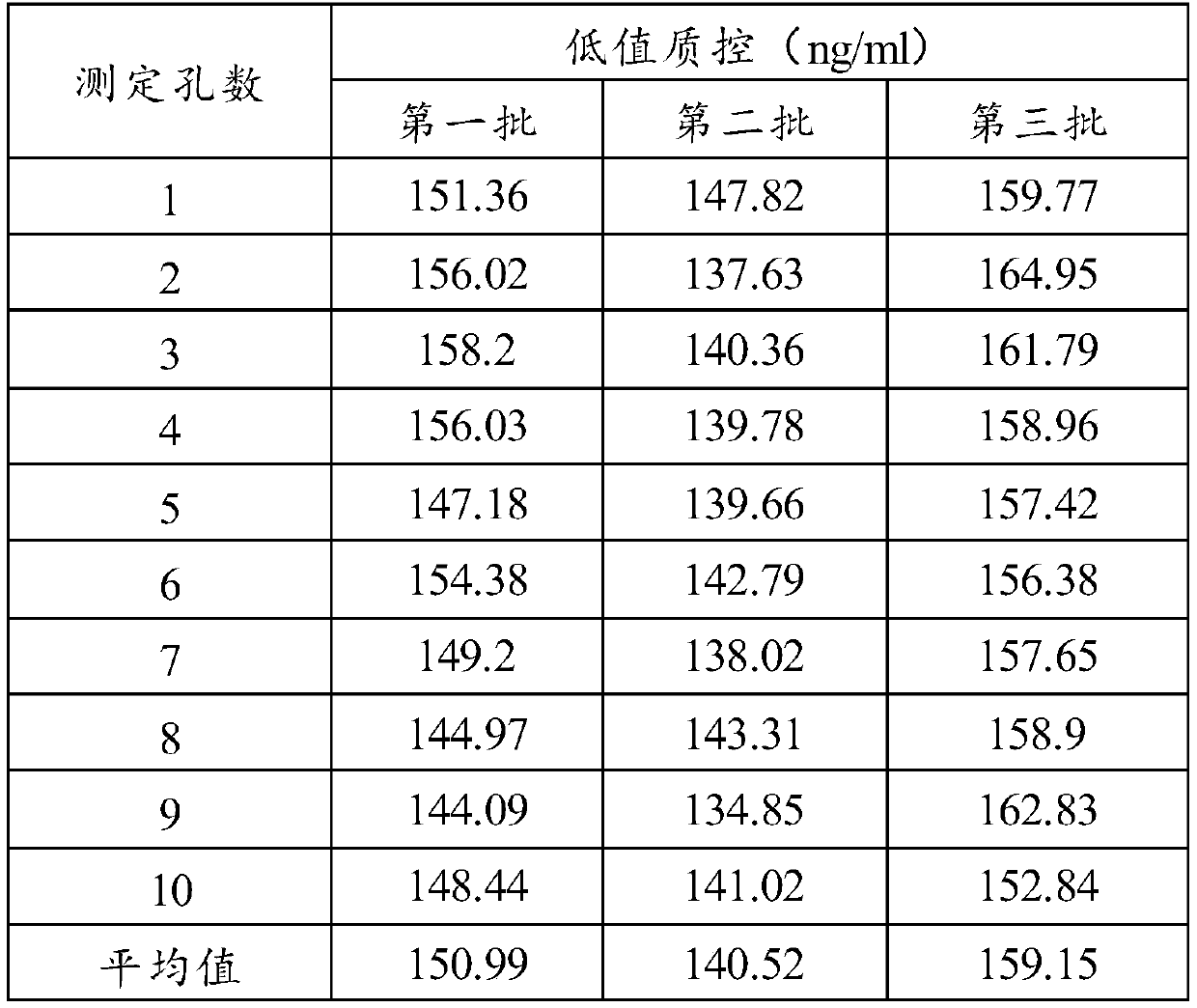
[0071] The performance detection of embodiment 3 kits of the present invention
[0072] 1. Analytical specificity
[0073] Analytical specificity is mainly used to control that the detected substance of the kit is a specific substance rather than others, especially the test is aimed at substances with a similar structure to the tested substance, and the specificity of the antibody will be insufficient. error.
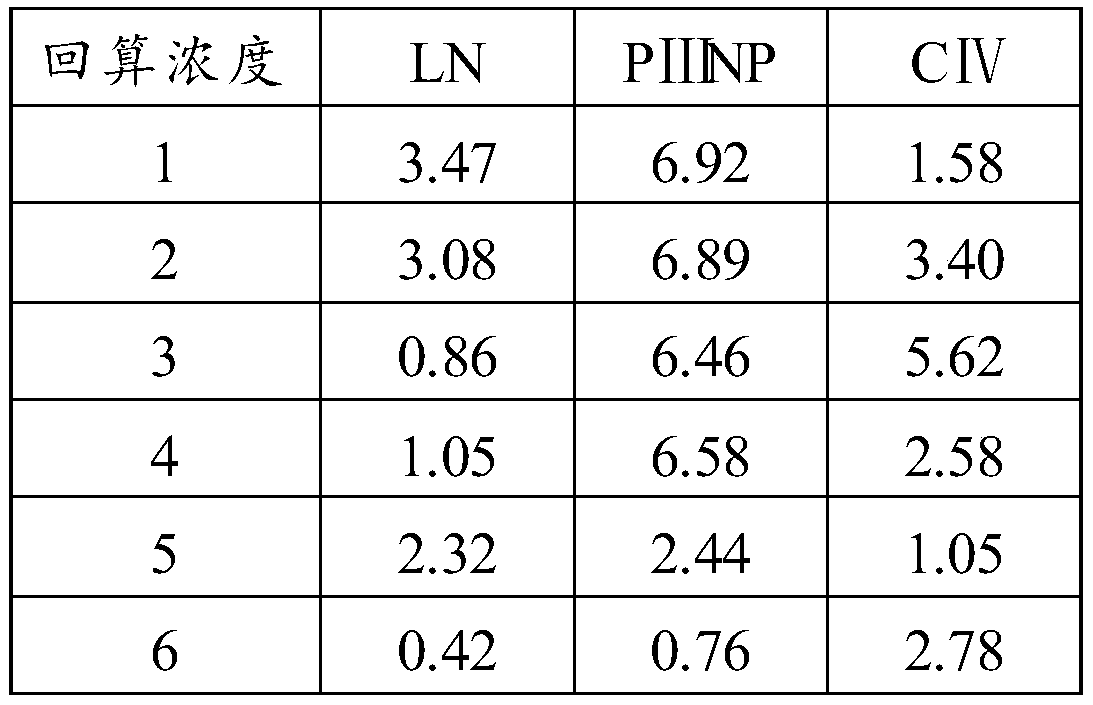
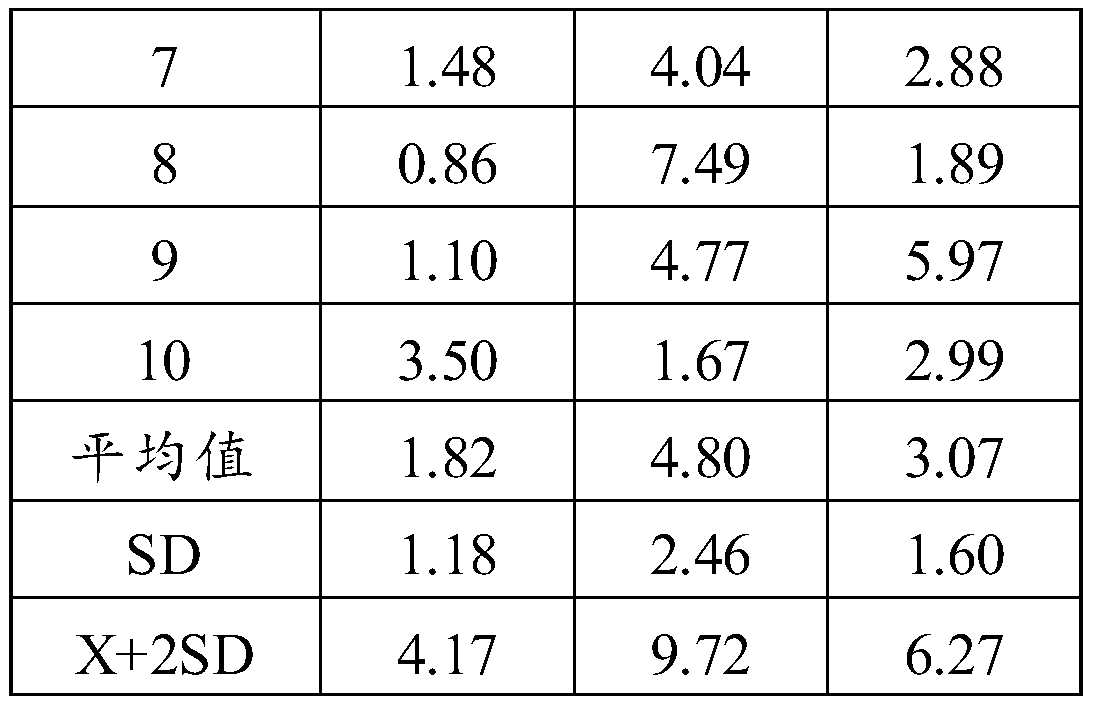
[0074] In order to avoid cross-reaction with the other three items of the liver fibrosis item, LN, PⅢNP, and CⅣ should be used as the indicators for detecting the specificity of HA. Since the reagents used are all from the same manufacturer, the test method is to use a batch of products to measure 1000ng / ml LN and CⅣ, and 100ng / ml PⅢNP respectively, and observe whether there is cross-reactivity. The experimental results are shown in Table 1 below.
[0075] Table 1 Specificity detection results
[0076]
[0077]
[0078] It can be seen from the test results in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com