Chlorpromazine derivative, preparation method thereof and chlorpromazine detection reagent
A detection reagent, chlorpromazine technology, applied in the field of chlorpromazine detection reagent, chlorpromazine derivatives and its preparation, can solve the problems of slow speed, complicated operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1. The synthesis of chlorpromazine derivative
[0106] The chemical structure of chlorpromazine derivative is as shown in formula I:
[0107]
[0108] Formula I.
[0109] The concrete route of the synthetic method of above-mentioned chlorpromazine derivative is as follows:
[0110] .
[0111] Concrete synthetic steps are as follows:
[0112] 1. Synthesis of compound 3
[0113]
[0114] Dissolve 10 g of Compound 1 in 120 mL of DMF, then add 5.15 g of t-BuOK to prepare a reaction solution, then stir the reaction solution at 60°C for 1 hour, and cool the reaction solution to 25°C after the stirring, and then Add 12 g of compound 2, then stir the mixture overnight at room temperature, add 200 mL of purified water after the stirring, then extract the reacted solution with 300 mL of DCM, repeat 3 times, combine the extracted organic phases and concentrate , the concentrated product was purified by a silica gel column (PE:EA=20:1)...
Embodiment 2
[0132] Embodiment 2. Preparation of chlorpromazine homogeneous enzyme immunoassay reagent
[0133] The preparation method of chlorpromazine homogeneous enzyme immunoassay reagent, concrete steps are as follows:
[0134] (1) Add 0.25% bovine serum albumin, 50mmol / L glucose-6-phosphate and 50mmol / L oxidized form of nicotinamide adenine dinucleotide to 50mmol / L Tris buffer and stir to dissolve to make R1 buffer, Then add anti-chlorpromazine specific antibody 1 to the above-mentioned R1 buffer at a volume ratio of 1:1500 and mix well, then adjust the pH to 8.0 with 6 mol / L hydrochloric acid to prepare the R1 reagent;
[0135] (2) Add 0.25% bovine serum albumin into 100mmol / L Tris buffer and stir to dissolve to make R2 buffer, then add chlorpromazine glucose-6-phosphate dehydrogenase labeled conjugate at a volume ratio of 1:3000 Add it to the above R2 buffer and mix well, then adjust the pH to 7.6 with 6 mol / L hydrochloric acid to make R2 reagent.
[0136] The preparati...
Embodiment 3
[0145] Embodiment 3. The preparation of chlorpromazine calibrator
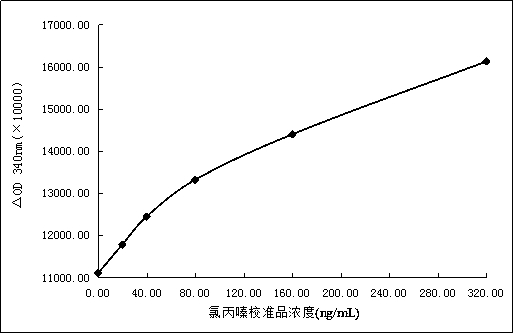
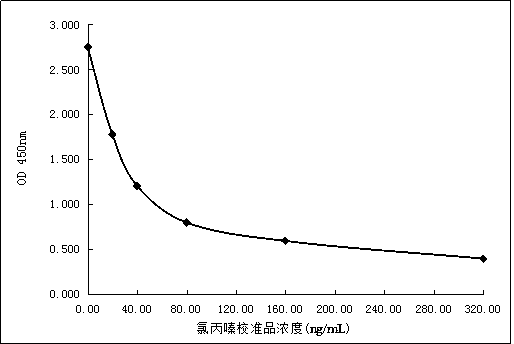
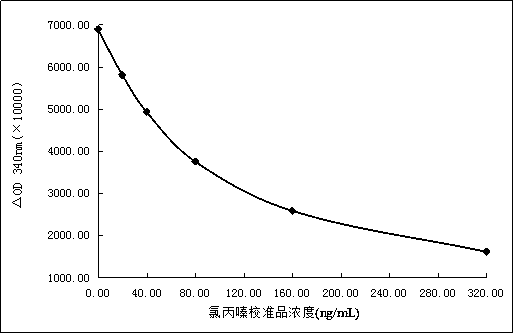
[0146] Add the pure powder of chlorpromazine to 5 parts of Tris-HCl buffer solution with a concentration of 50mmol / L pH=7.2, stir and dissolve until the final concentrations are 0ng / mL, 20ng / mL, 40ng / mL, 80ng / mL , 160ng / mL, 320ng / mL, and then add 0.5% sodium chloride, 1.0% bovine serum albumin, 0.75% ethylenediaminetetraacetic acid, 0.05% sodium azide to each solution , Stir evenly, that is the chlorpromazine calibration standard (6 concentrations).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com