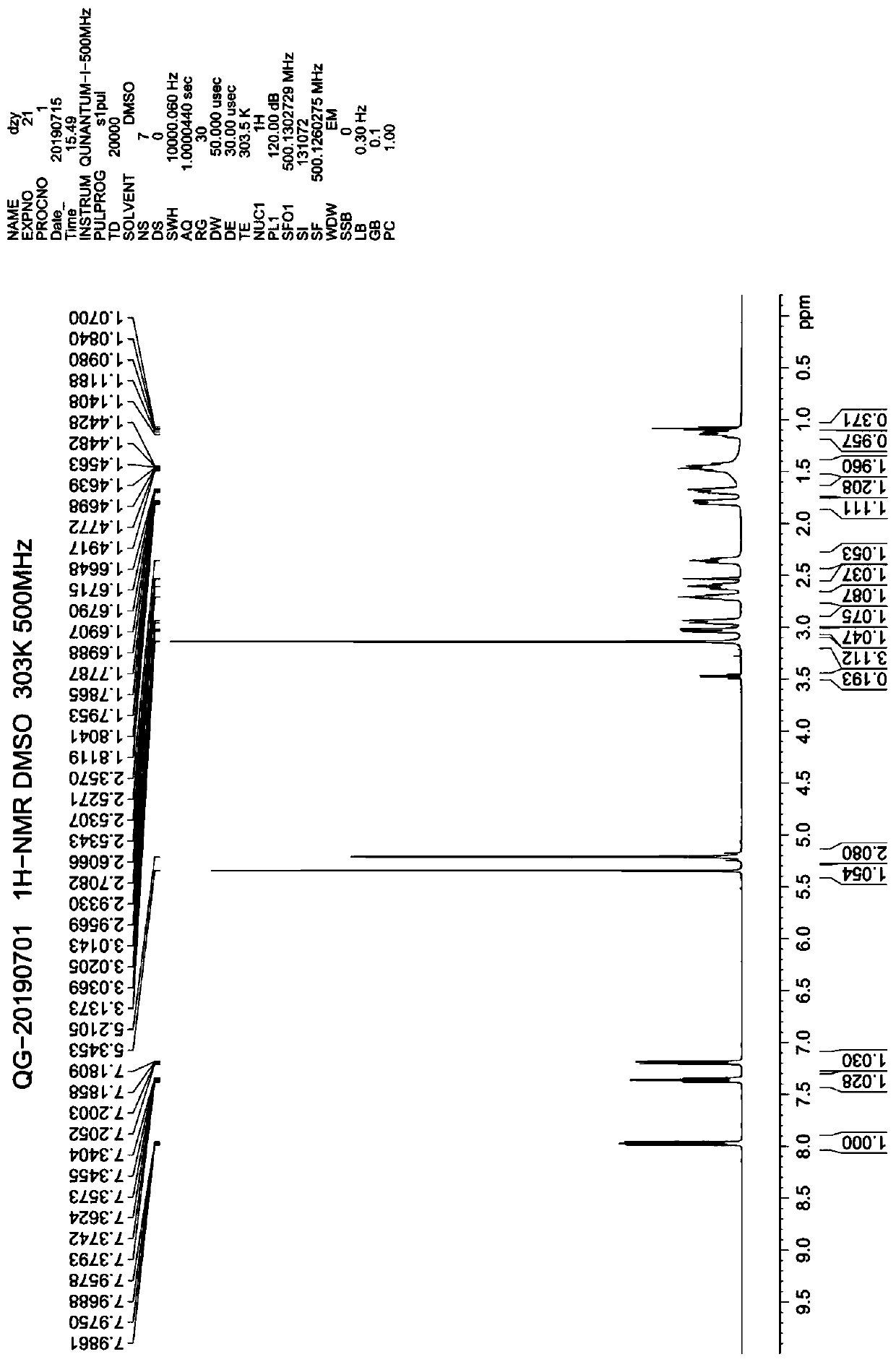
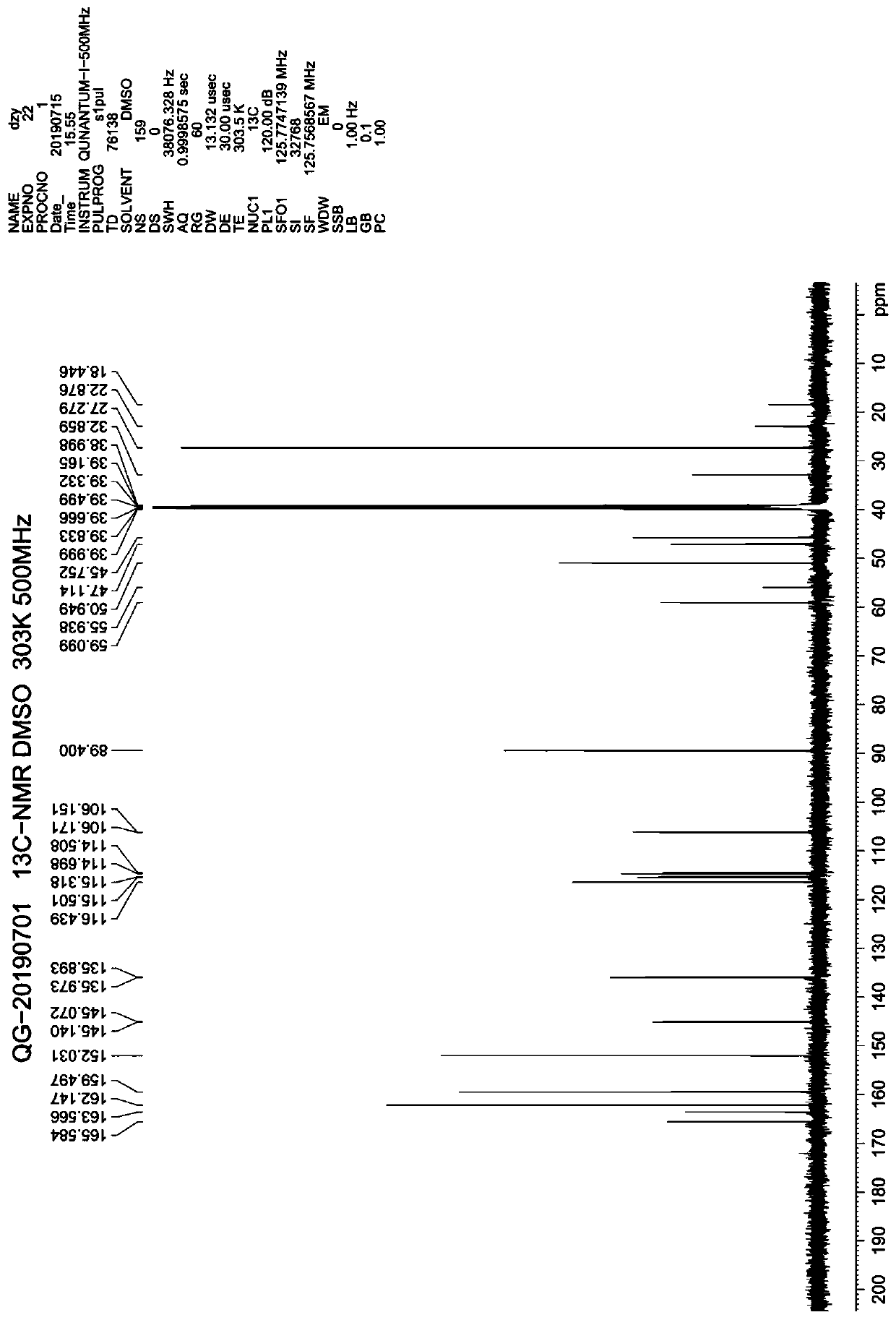
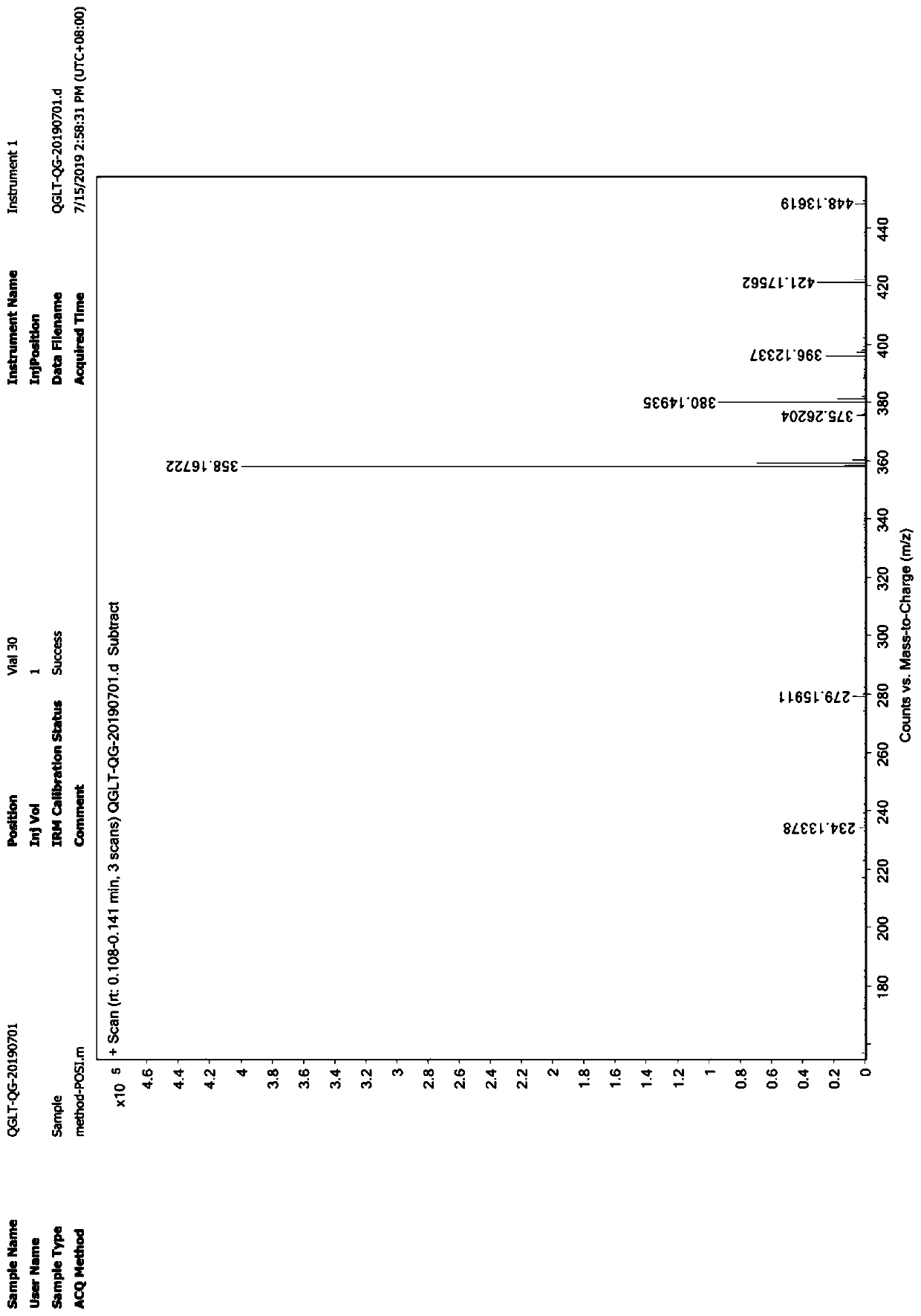
Preparation method of trelagliptin succinate
A technology of troxagliptin succinate and intermediates, applied in the field of pharmaceutical production, can solve the problems of poor economic benefits and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 300g of toluene and 75g of 3-aminopiperidine into a 500mL four-necked reaction flask, add 83.5g of benzaldehyde under stirring, then raise the temperature and reflux with water until no water comes out, and the temperature of the material liquid reaches above 110°C. Bring to room temperature, filter, wash the filter cake with an appropriate amount of drinking water, and filter to dry to obtain intermediate III. After drying, 136.5 g of intermediate III is obtained as a dry product.
[0033] Add 600g of DMF, 150g of II, and 141g of anhydrous potassium carbonate to a 1L four-necked reaction flask, raise the temperature to 60-70°C, put in 96g of intermediate III, then keep it warm for 8 hours, and filter at room temperature. Distill DMF to dryness under pressure to obtain a reddish-brown oily substance, which is the condensation intermediate IV.
[0034] Then add 900ml of drinking water, after stirring, convert it into a 2L four-necked reaction flask, add 186g of hydro...
Embodiment 2
[0043] Add 300g of toluene and 50g of 3-aminopiperidine into a 500mL four-necked reaction flask, add 63.6g of benzaldehyde under stirring, then heat up and reflux with water until no water comes out, and the temperature of the material liquid reaches above 110°C. Bring to room temperature, filter, wash the filter cake with an appropriate amount of drinking water, and filter to dry to obtain intermediate III. After drying, 88.9g of intermediate III is obtained as a dry product.
[0044] Add 480g of DMF, 80g of intermediate II, and 83g of anhydrous potassium carbonate to a 1L four-necked reaction flask, raise the temperature to 60-70°C, put in 51.3g of intermediate III, keep it warm for 4 hours, and filter it at room temperature. DMF was distilled under reduced pressure in the reaction flask to dryness, then 900ml of drinking water was added, and after stirring, it was converted into a 2L four-necked reaction flask, and 166g of hydrochloric acid with a mass content of 30% was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com