Polypeptides and applications for protein surface immobilization
A fusion protein and sequence technology, applied in the protein field, can solve the problems of non-site-specific binding, increase the time and cost of the immobilization process, damage the protein, etc., and achieve the effect of enhancing binding affinity, eliminating steric hindrance and high binding strength.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
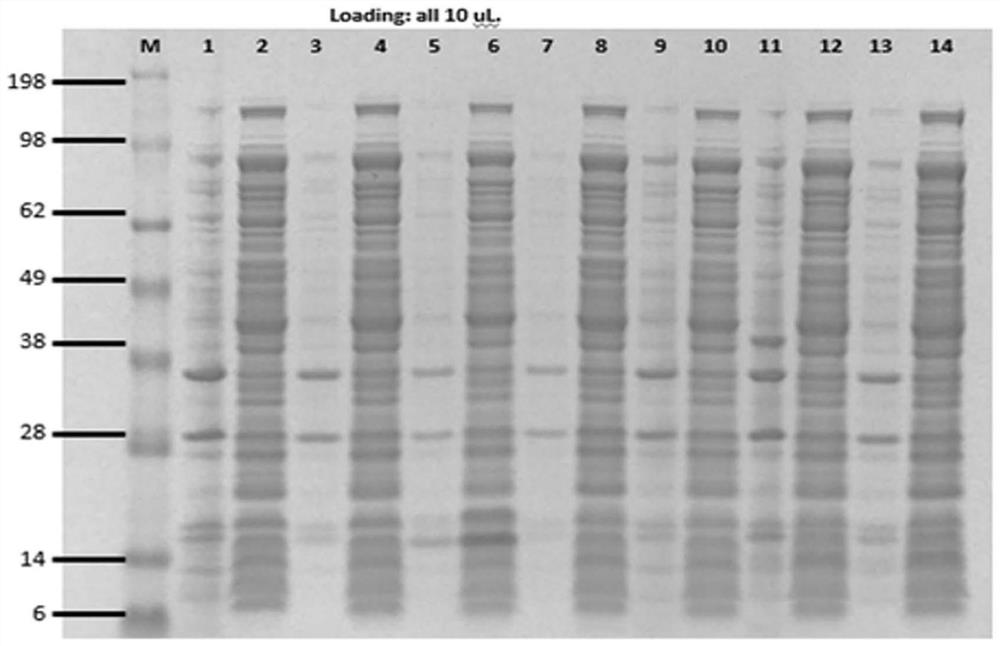
[0055] Example 1 Preparation of silica-binding peptide anti-CEA nanobody fusion protein
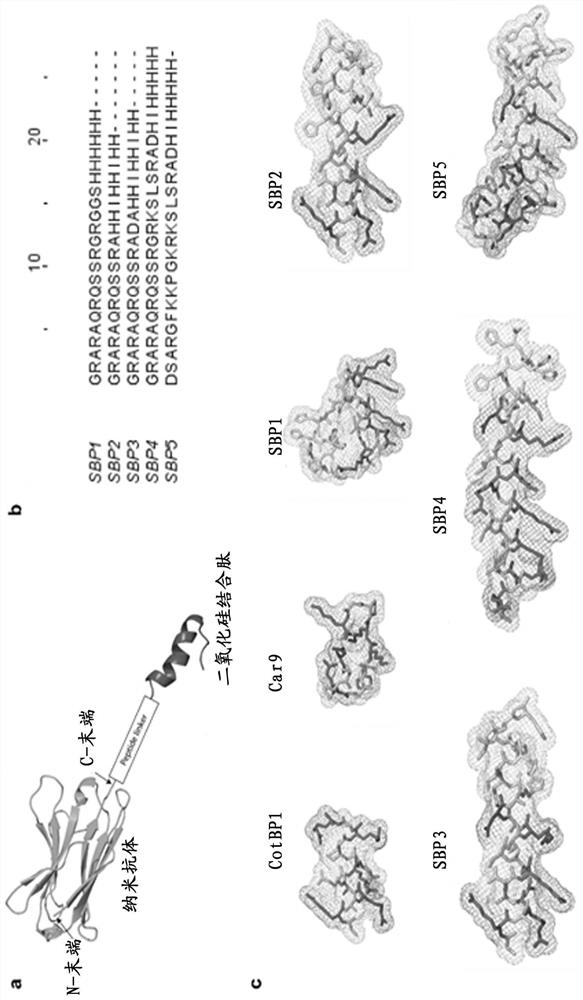
[0056] 1. Construction of Silica Binding Peptide Anti-CEA Nanobody Fusion Protein
[0057] The silica-binding peptide was reconstituted into an anti-CEA heavy chain antibody (clone number 2D5, 11C12) isolated from immunized llamas following the procedure described in WO 94 / 25591. For information on 2D5 heavy chain antibody, see Chinese patent application CN201711358747.4). For information on the 11C12 heavy chain antibody, see Chinese patent application CN201710120052.6. The sequences of the silica-binding peptides are shown in SEQ ID NO. 9-13, which are named SBP1-5 in the present invention. In addition, the present invention also uses the silica-binding peptide CotB1P from the prior art (Abdelhamid, M.A., Motomura , K., Ikeda, T., Ishida, T., Hirota, R., & Kuroda, A. (2014). Affinity purification of recombinant proteins using a novel silica-binding peptide as a fusion tag. Applied mic...
Embodiment 2
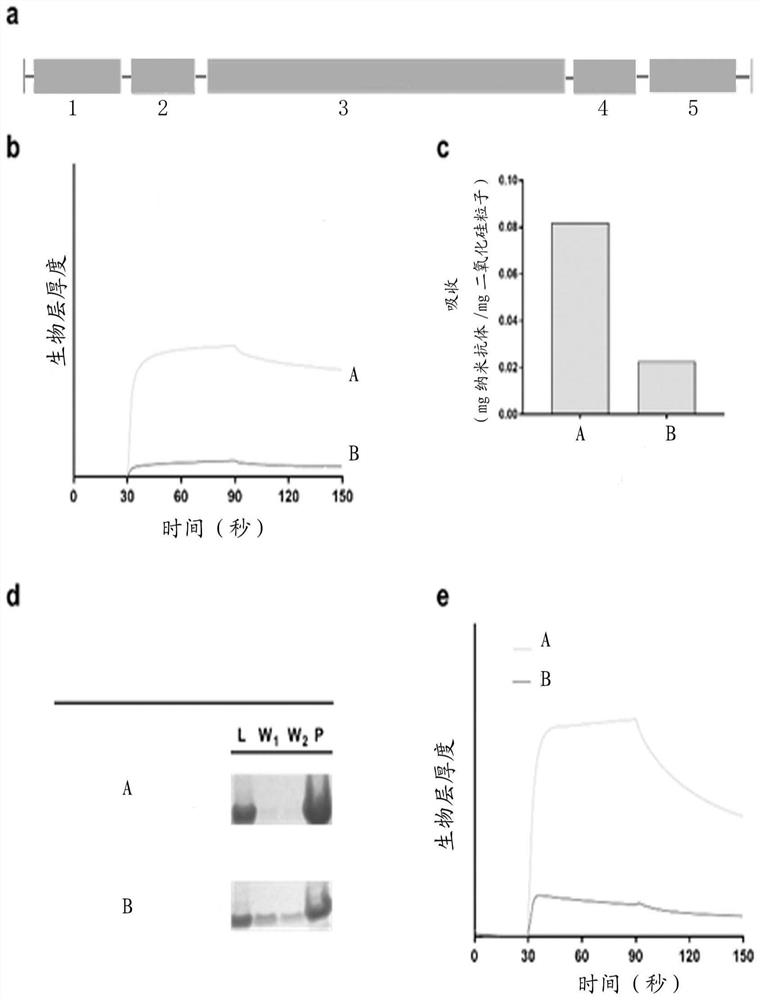
[0084] Example 2 Binding Kinetics Determination of the Interaction of Tailed Antibody Fragments with Quartz Surfaces
[0085] A novel biosensor (FORTEBIO) was placed in a 70°C water bath oxidizing solution (H 2 O 2 , a mixture of water and ammonia) for 30 minutes and washed with ultrapure water to prepare the quartz surface. The prepared quartz sensor was used to measure the binding kinetics with a BLItz biolayer interferometer (FORTEBIO).
[0086] Specific steps for the determination of binding kinetics:
[0087] The binding kinetics of the silicon-based bound Nanobody constructs were determined using biolayer interferometry (BLItz (FORTEBIO)). Oxidize liquid at 70°C (H 2 The silicon-based surface of the end of the quartz biosensor was incubated in a water bath for 30 minutes to prepare the silicon-based surface, and then washed with MilliQ water. Quartz biosensors were stored in 20 v / v% ethanol and pre-equilibrated in PBS for 10 minutes before use. Unless otherwise ind...
Embodiment 3
[0093] Example 3 Silica immobilization of tailed anti-CEA heavy chain antibodies
[0094] The silica nanoparticles and microspheres were ultrasonically dispersed in PBS for 5 minutes (Qsonica Q125). The purified heavy chain antibody is added to the dispersed silica nanoparticles or microspheres. After a short vortex, incubate at room temperature for 30 minutes. The mixture was then centrifuged at 14800 rpm for 5 minutes to separate the particles. Wash twice with PBS and collect supernatant from each wash step. Heavy chain antibodies immobilized in particles or supernatants were quantified by SDS-PAGE and absorbance at 280 nm.
[0095] Published silica-binding peptides (such as CotB1p, ibid.) have failed to identify polyhistidine as a key residue for enhancing silica-binding affinity by forming hydrogen bonds with surface silanols. In the present invention, at least one histidine is added to facilitate the formation of hydrogen bonds. The dissociation constant of one of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com