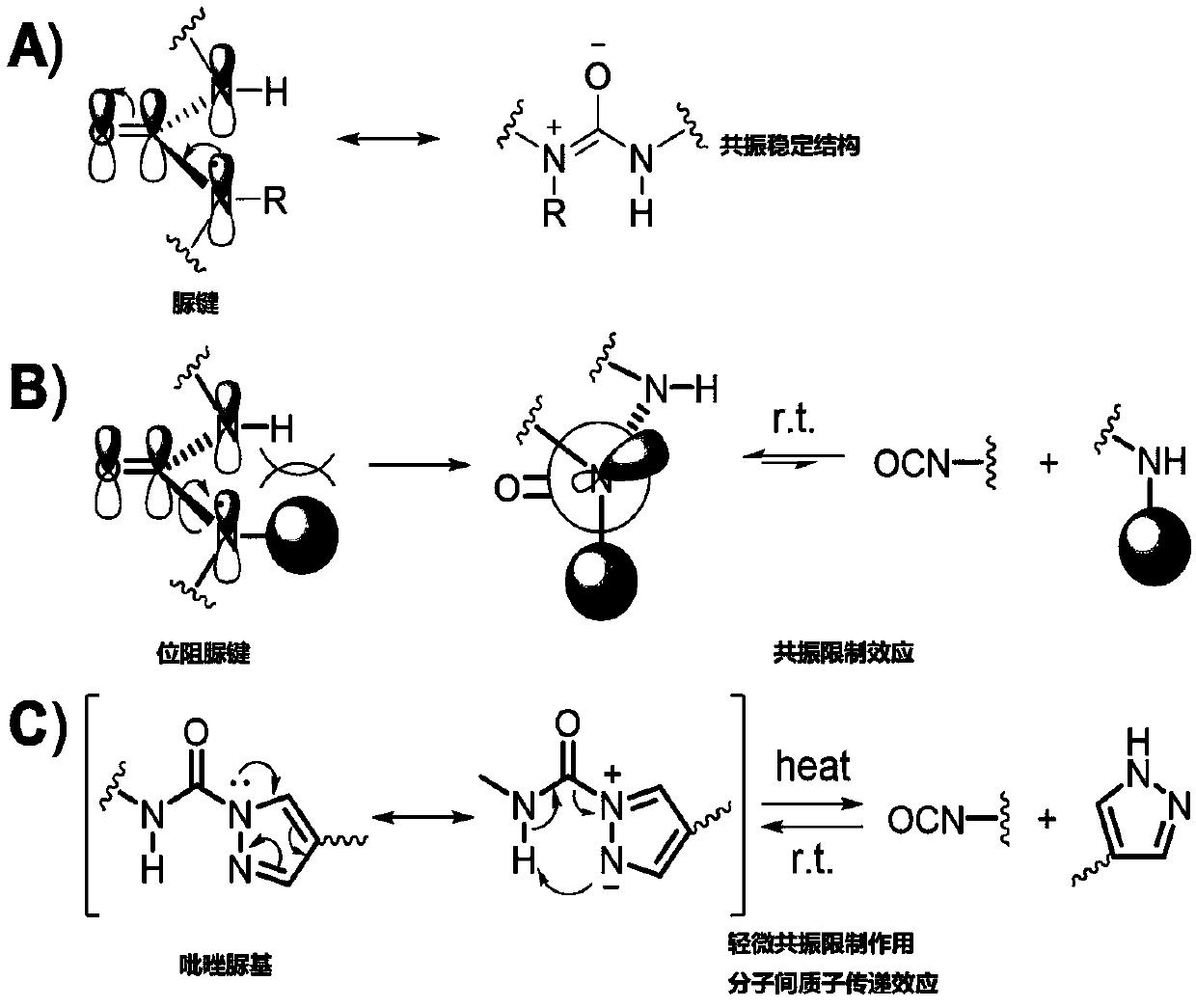
Pyrazolyl ureido-based polyurea urethane, and preparation method and application thereof
A technology of pyrazole urea group and urea urethane, applied in the field of polyurea urethane material, can solve the problems of restricting the application of dynamic sterically hindered urea bonds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
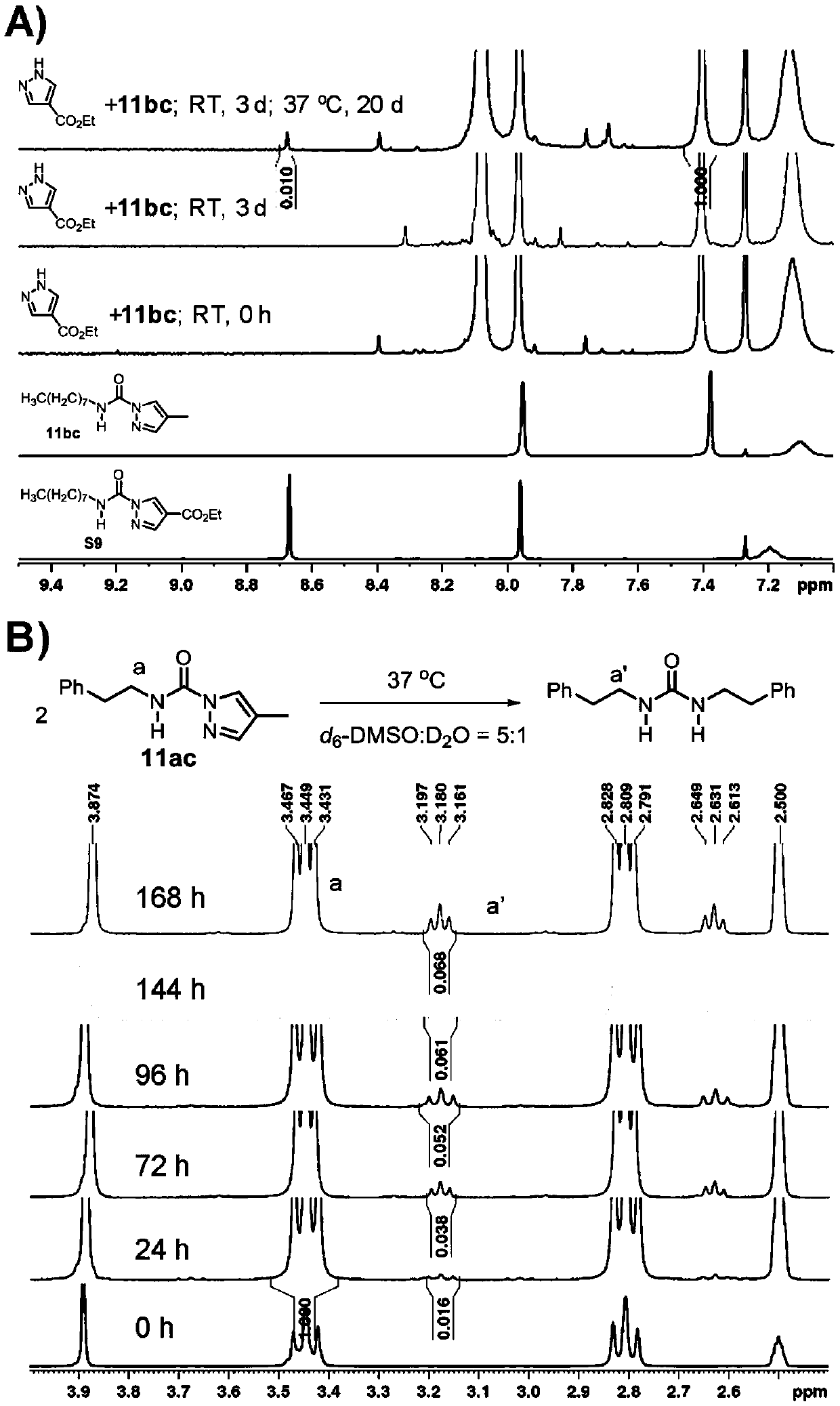
Embodiment 1
[0087] The preparation of embodiment 1 containing pyrazole carbamide group compound
[0088]
[0089] 4-Methylpyrazole 1c was dissolved in 5 mL of anhydrous DCM, n-octyl isocyanate was added, reacted at room temperature for 1 h, concentrated, and column chromatography (n-hexane / EtOAc=80:1 to 30:1) gave white solid 11bc (3.37 g, 99% yield).
[0090] The structural confirmation data are as follows: 1 H NMR (300MHz, CDCl 3 ,ppm)δ7.95(s,1H),7.38(s,1H),7.10(br,s,1H),3.37(q,J=6.7Hz,2H),2.07(s,3H),1.63-1.54 (m,2H),1.31-1.25(m,10H),0.86(t,J=6.5Hz,3H); 13 C NMR (75MHz, CDCl 3 ,ppm)δ149.8,142.9,126.6,118.6,40.2,31.7,29.6,29.1,29.1,26.7,22.5,14.0,8.7; IR(neat,cm -1 )3352,3121,2951,2921,2852,1707,1525,1468,1389,1271,1010,836,754; ESI-HRMS calculated value C 13 h 23 N 3 NaO[M+Na] + :260.1733, experimental value: 260.1735, error ((calculated value-experimental value) / calculated value): -0.8ppm.
[0091]
[0092] The synthesis operation was similar to 11bc to obtain 11bd (99%...
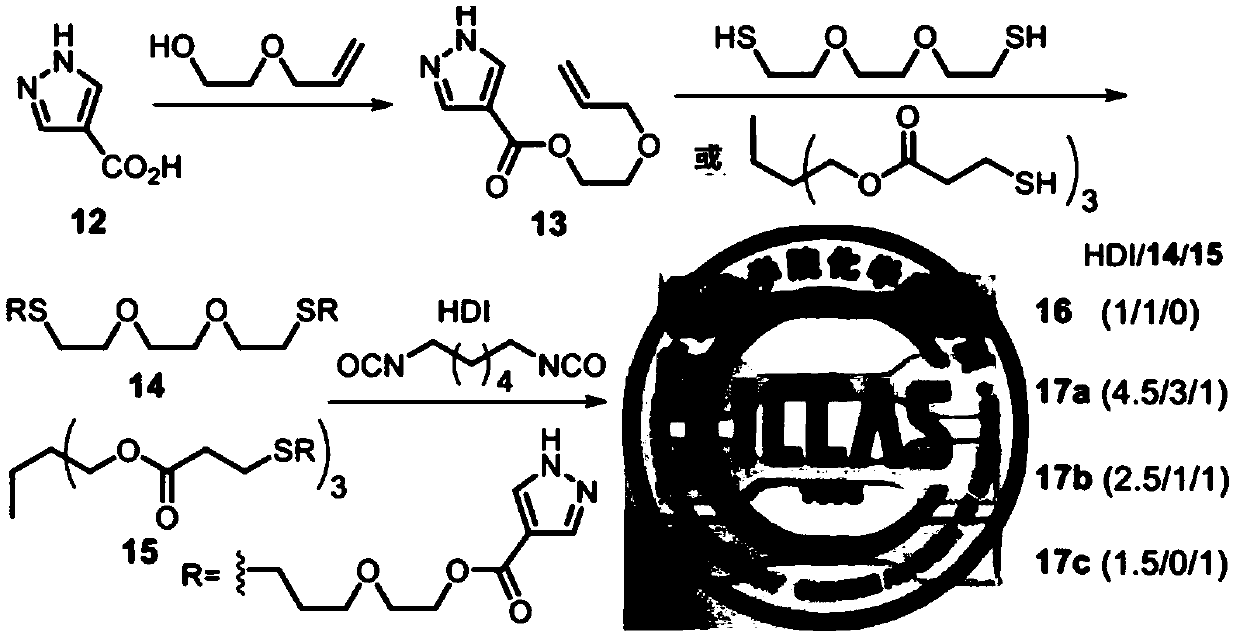
Embodiment 2
[0115]
[0116] 4-Pyrazolecarboxylic acid 12 (29.97g, 262mmol) and 2-allyloxyethanol (35.53g, 341mmol) were dissolved in 180mL of anhydrous DMF, and EDCI (55.25g, 288mmol) and DMAP (12.8g, 105mmol) were added successively ), stirred at room temperature for 3d. Concentrate the solution at 70°C to remove DMF, then add 200 mL of water, Et 2 O (3×300mL) was extracted, the organic phases were combined, and successively washed with 10wt% citric acid (4×100mL), saturated NaHCO 3 Wash with saturated brine, Na 2 SO 4 Dry and concentrate. Distilled under reduced pressure at 110°C for 20 min to remove 2-allyloxyethanol to obtain light yellow liquid compound 13 (31.05 g, 60% yield).
[0117] The structural confirmation data are as follows: 1 H NMR (300MHz, CDCl 3 ,ppm)δ11.52(br,s,1H),8.07(s,2H),5.97-5.84(m,1H),5.29(dq,J=17.2Hz,J=1.6Hz,1H),5.20(dq , J=10.4Hz, J=1.3Hz, 1H), 4.42(t, J=4.8Hz, 2H), 4.06(dt, J=5.6Hz, J=1.3Hz, 2H), 3.75(t, J=4.8 Hz,2H); 13 CNMR (75MHz, CDCl 3 , ppm)...
Embodiment 3
[0124] Embodiment 3 Preparation of polyureaurethane based on pyrazole urea group structure
[0125]
[0126] Dissolve the dipyrazole compound 14 (2.501 g, 1 equivalent) prepared in Example 2 above in 6 mL of anhydrous CHCl 3 , HDI (0.725g, 1 equivalent) was added and reacted at room temperature for 10h. The reaction solution was evaporated at room temperature for 16 hours, and then vacuumed at 70° C. for 48 hours. The obtained white solid 16 was hot-pressed for 30 minutes (130° C., 10 MPa) to obtain a complete film sample, which was stored in a desiccator.
[0127] The structural confirmation data are as follows: 1 H NMR (300MHz, CDCl 3 ,ppm)δ8.70(s,2H),7.98(s,2H),7.25(t,J=5.9Hz,2H),4.41(t,J=4.5Hz,4H),3.72(t,J=4.7 Hz,4H),3.65-3.56(m,12H),3.44(q,J=6.7Hz,4H),2.70(t,J=6.9Hz,4H),2.63(t,J=7.2Hz,4H), 1.91-1.82(m,4H),1.70-1.62(4H),1.48-1.42(4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com