Application of soluble CD146 as blood-brain barrier injury marker in central nervous system diseases
A central nervous system, soluble technology, applied in biological testing, chemiluminescence/bioluminescence, analysis by making materials react chemically, etc., can solve the problems of elevated, insufficient specificity, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of paired monoclonal antibodies
[0031] Referring to Chinese patent application numbers 200810057260.7, 201210394856.2 and 99107586, monoclonal antibodies AA1, AA4, and AA98 or their derivatives or antigen-binding fragments were prepared using hybridoma technology. The antigenic epitope it binds to, the CDR sequence of the heavy chain variable region and the CDR sequence of the light chain variable region contained in each of them are as described above, and the strains that secrete them are the strains with the preservation number of CGMCC NO.2310 and the preservation number of It is the strain of CGMCC NO.2311 and the strain of preservation number CGMCC NO.0491.
Embodiment 2
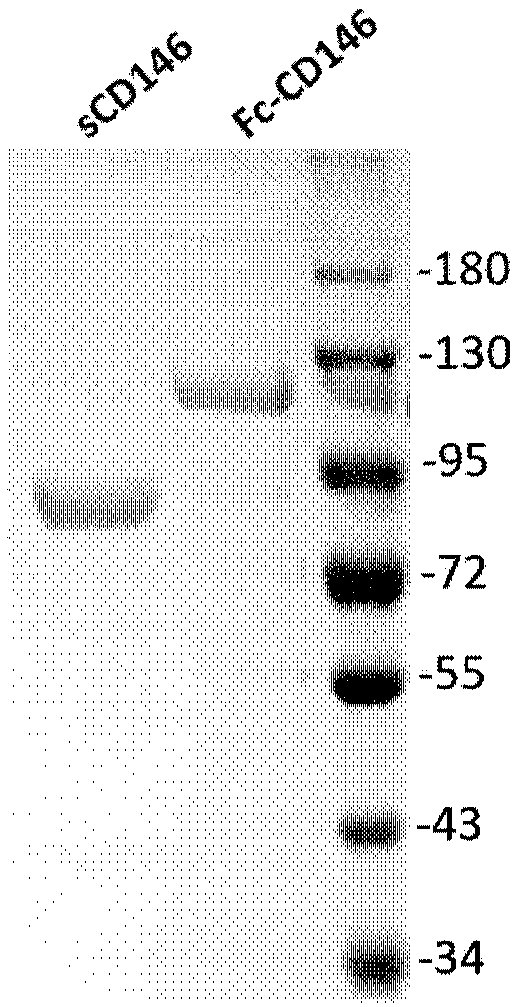
[0032] Example 2: Expression and purification of standard sCD146 protein
[0033] sCD146 is a recombinantly expressed extracellular region protein of human CD146 molecule (amino acids at positions 22-559 in SEQ ID NO: 1, refer to Chinese Patent Application No. 200810057260.7, 201210394856.2). The CD146 cDNA (SEQ ID NO: 2) sequence with signal peptide, His-tag, strep-tag, and HRV3C recognition sequence at the N-terminal was cloned into the pcDNA3.1 vector (Invitrogen), and the plasmid was transfected into CHO Lec 3.2.8.2 Cells (ATCC) were cultured in DMEM medium, cells with higher protein expression levels were screened, and the culture was expanded and the culture supernatant was collected. The cell culture supernatant was further concentrated and affinity-purified with Ni-NTA (Qiagen) and strep-tactin (IBA) to obtain a fusion protein containing His-tag and strep-tag with higher purity. After digestion with HRV3C (Sino Biological) protease and Ni-NTA affinity purification to ...
Embodiment 3
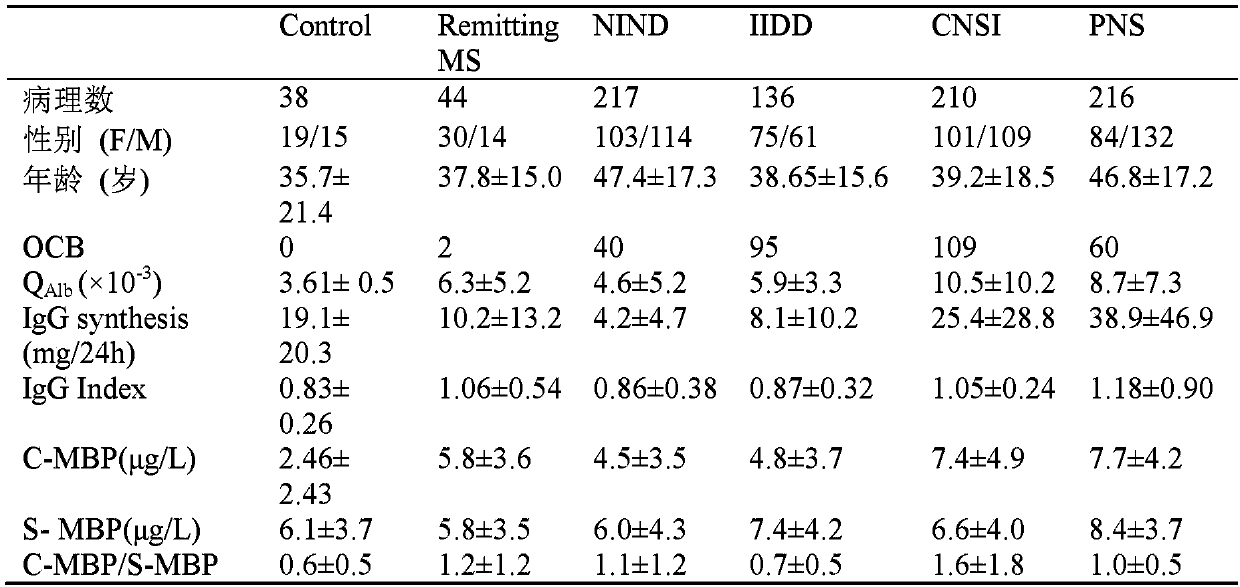
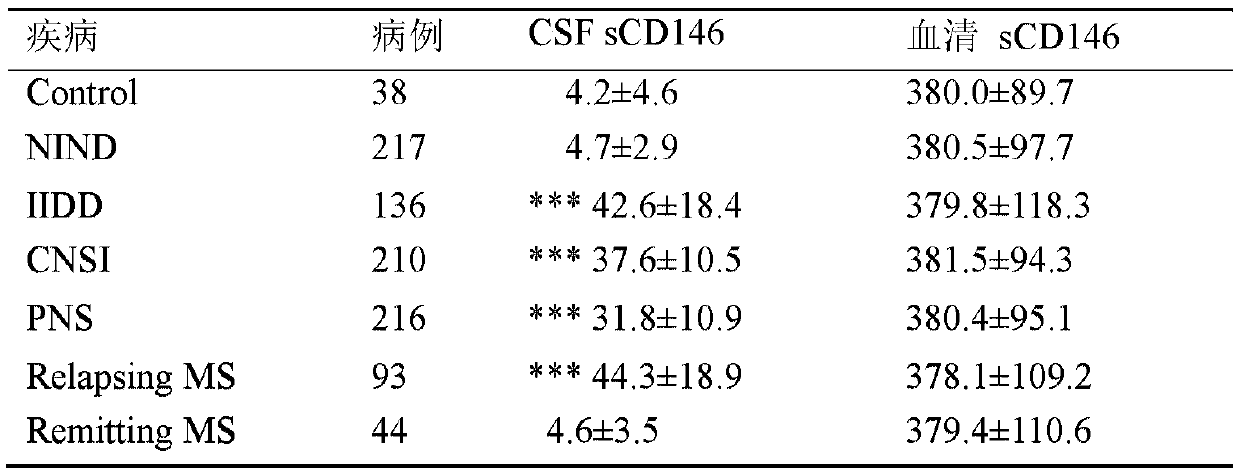
[0034] Embodiment 3: clinical sample collection and classification
[0035]The inventor collected 861 pairs of cerebrospinal fluid and serum samples from patients with central nervous system diseases who were treated at the First Affiliated Hospital of Peking University and Beijing Anzhen Hospital from 2011 to 2018. The cerebrospinal fluid sample was obtained by lumbar puncture, centrifuged at 1000rpm at 4°C for 5 minutes, and the supernatant was aliquoted and stored at -80°C. . All patients had the right to know that their own serum and cerebrospinal fluid samples were used in this retrospective study, and were approved by the ethics committees of the First Affiliated Hospital of Peking University and Beijing Anzhen Hospital. According to the clinicopathological information of the patients, the inventors divided the patients included in the analysis into 6 groups, namely the control group (control), remitting multiple sclerosis (Remitting MS), nervous system non-inflammatory...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com