Application of lappaconitine or 12-epi-lappaconitine in preparation of medicine for treating leukemia
A technology of eugenide and leukemia, applied in the field of medicine, can solve the problems of high mortality rate of leukemia patients and poor treatment strategies, and achieve the effects of promoting apoptosis, inhibiting proliferation and having broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Anti-leukemia cell proliferation activity experiment:
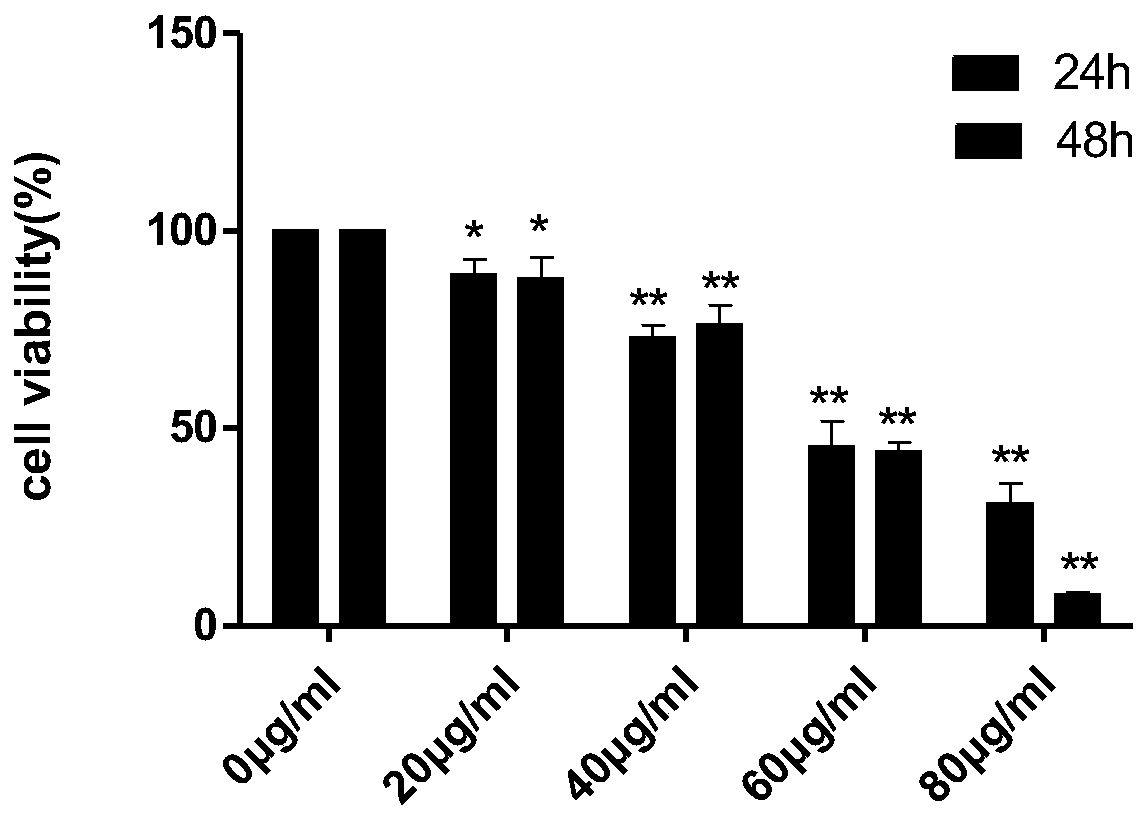
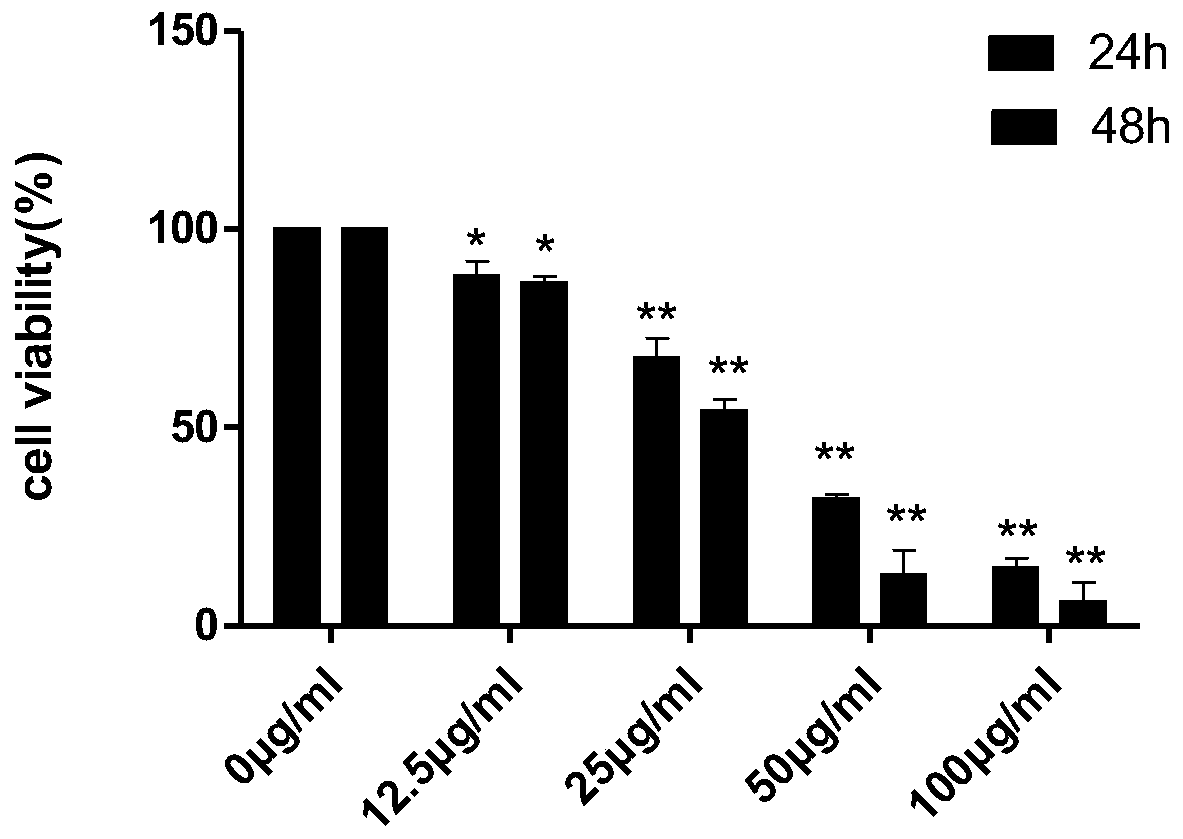
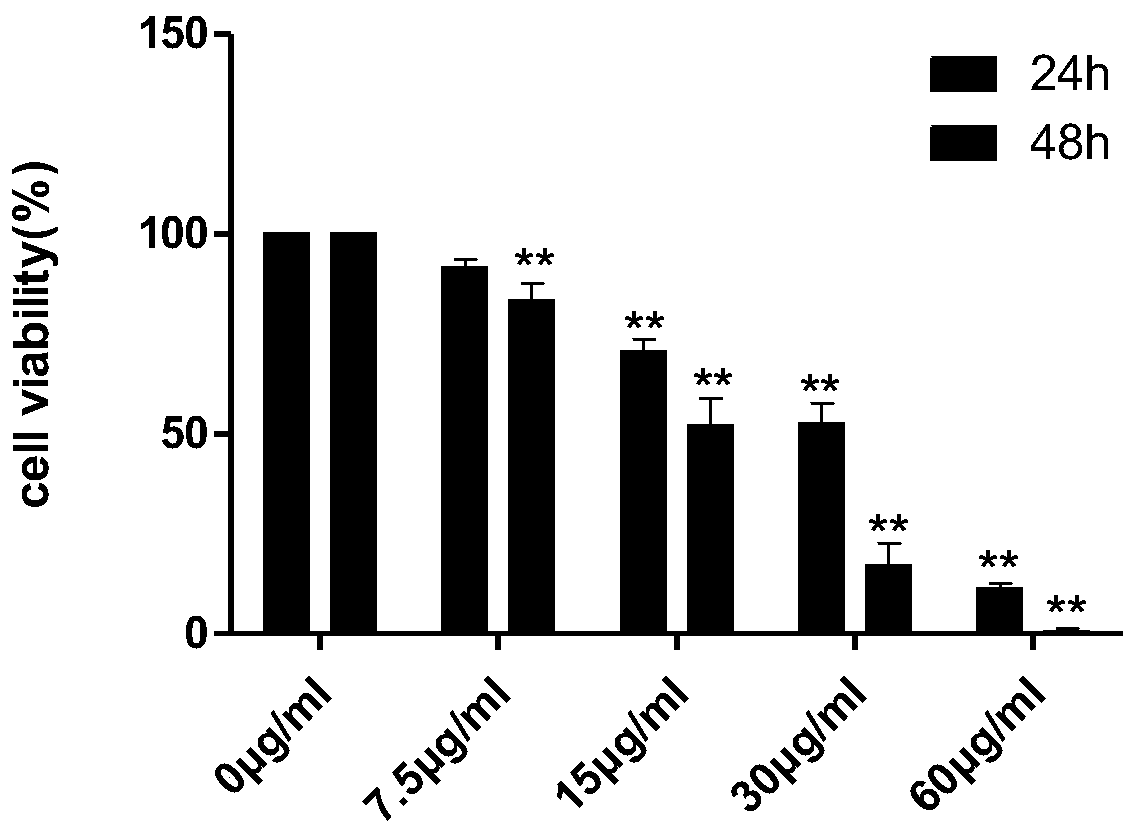
[0045] CCK-8 method was used to detect the anti-K-562 and HL-60 cell activity of oleucine and 12-epi-meurine.
[0046] (1) Drug preparation
[0047] The electronic balance weighs the appropriate amount of oleucine and 12-epi-meurine, and DMSO completely dissolves the drug.
[0048] (2) Solvent preparation
[0049] The preparation of medium: according to the preparation of high glucose DMEM medium:serum:double antibody=100:10:1
[0050] Preparation of PBS: Weigh 8g of NaCl, 10.2g of KC, 1.42g of Na2HPO4, and 0.27g of KH2PO4 with an electronic balance; add about 800ml of deionized water, fully stir and dissolve on a magnetic stirrer, and then set the volume to 1L. Autoclave at 120°C for 30 minutes and place in a 4°C refrigerator.
[0051] Preparation of cryopreservation solution: Prepare according to serum:DMSO=9:1, and use immediately after preparation.
[0052] (3) Cell culture
[0053] In each experiment of th...
Embodiment 2
[0074] Promoting leukemia cell apoptosis experiment:
[0075] The apoptosis of K-562 and HL-60 cells detected by flow cytometry
[0076] (1) Drug preparation
[0077] The electronic balance weighs the appropriate amount of oleucine and 12-epi-meurine, and DMSO completely dissolves the drug.
[0078] (2) Seed board
[0079] ① K-562 cells: Collect K-562 cells in the logarithmic growth phase, inoculate them into 6-well plates at a density of 6×105 / well, and store them at 37°C, 5% CO 2 Routinely culture in the incubator for several hours until the cells are stable.
[0080] ②HL-60 cells: Collect HL-60 cells in the logarithmic growth phase, inoculate them into 6-well plates at a density of 1×106 / well, and store them at 37°C, 5% CO 2 Routinely culture in the incubator for several hours until the cells are stable.
[0081] (3) Dosing
[0082] ① K-562 cells: After the cells are stabilized, add oleucine (40, 60 μg / ml) and 12-epi-omeurine (25, 50 μg / ml). At 37°C, 5% CO 2 Routine...
Embodiment 3
[0095] Morphological detection of leukemia cell apoptosis:
[0096] The apoptosis of K-562 and HL-60 cells detected by Hoechst staining
[0097] (1) Seed board
[0098] ①K-562 cells: Take K-562 cells in the logarithmic growth phase, collect the cells, and divide them into 6×10 5 Inoculated into 6-well plates at a density of 1 / well and incubated at 37°C in 5% CO 2 Routinely culture in the incubator for several hours until the cells are stable. Set blank control group, low concentration group and high concentration group.
[0099] ②HL-60 cells: Take HL-60 cells in the logarithmic growth phase, collect the cells, and dilute 1×10 6 Inoculated into 6-well plates at a density of 1 / well and incubated at 37°C in 5% CO 2 Routinely culture in the incubator for several hours until the cells are stable. Set blank control group, low concentration group and high concentration group.
[0100] (2) Dosing
[0101] ① K-562 cells: After the cells are stabilized, add oleucine (40, 60 μg / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com