Oral insulin medicinal preparation and preparation method thereof
A technology for pharmaceutical preparations and insulin, applied in the field of biomedicine, can solve the problems of low bioavailability and achieve the effect of improving the absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of Oral Insulin Positioned Drug-Release-Coated Double-Layer Tablets
[0059] The preparation method of the oral insulin positioning drug-release coating double-layer tablet of the present embodiment includes the following steps:
[0060] (1) Preparation of insulin microemulsion freeze-dried powder
[0061] Get 1.0g insulin and be dissolved in the HCl of 80.0mL 0.01mol / L, and the drug solution is gradually added with ethyl oleate as oil phase, with mass ratio of 2:1 polyoxyethylene castor oil and polyethylene glycol- The mixture of 15 hydroxystearate is in the lipid mixture of surfactant (total 5.0g), and wherein, the mass ratio of oil phase and surfactant is 8:9, stirs into O / W type microemulsion; Slowly Add 0.1 mol / L NaOH solution dropwise to adjust the pH of the microemulsion to 5.5, and continue stirring for 2 hours; add lyophilization protective agents—mannitol and dextrin (2%, m / v) respectively, shake to dissolve completely, add water to dilu...
Embodiment 2
[0073] Example 2 Preparation of Oral Insulin Positioned Release Coated Double Layer Tablets
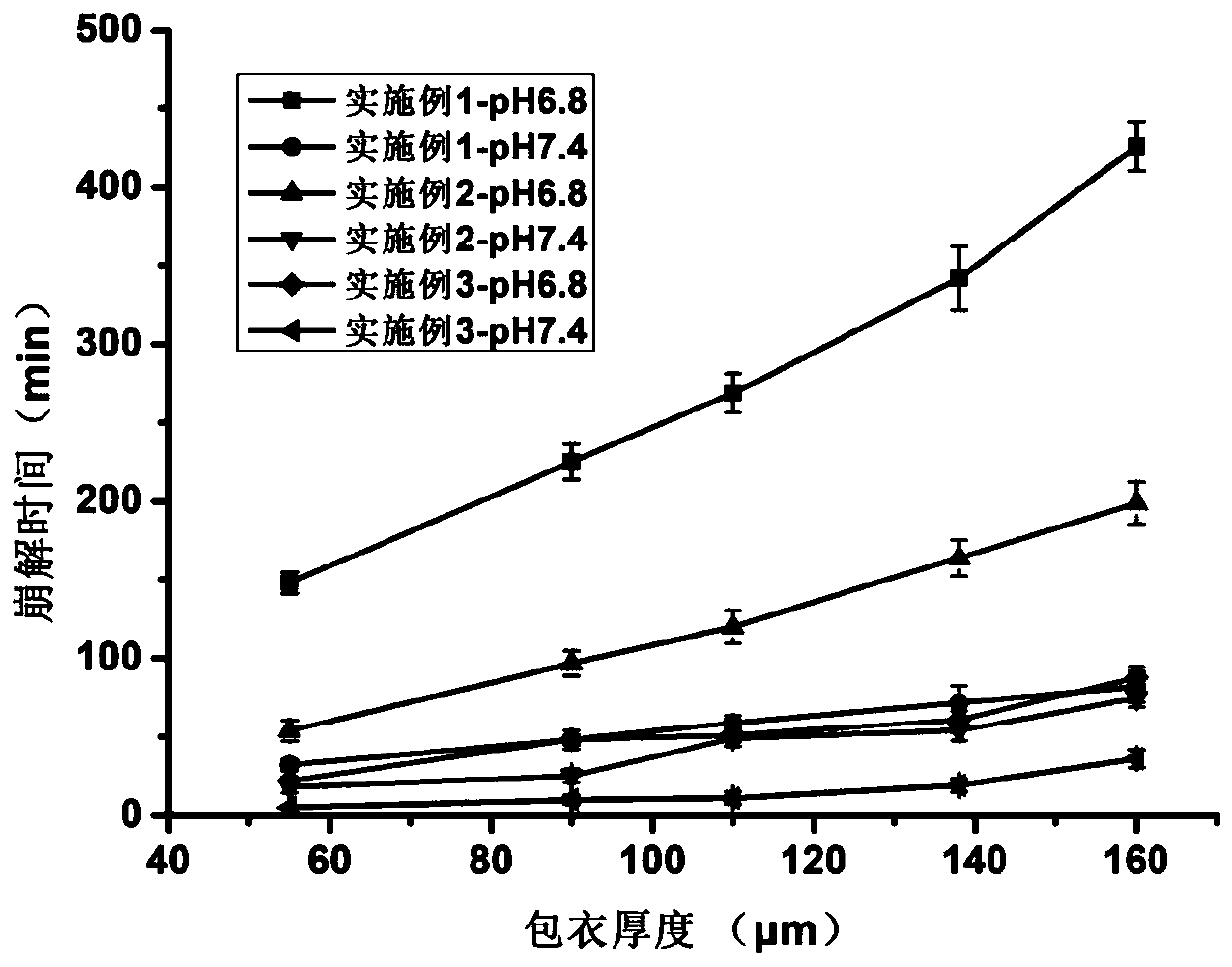
[0074] The preparation method of this example is the same as that of Example 1, the difference is that the drug-positioned drug release coating material of the back-coated double-layer tablet is prepared from a 95% ethanol solution with a mass ratio of Eudragit L100 and Eudragit S100 of 1:4. For a solution with a mass concentration of 10%, a series of tablets with different localized drug release coating thicknesses of 50, 90, 110, 140, and 160 μm were also prepared.
Embodiment 3
[0075] Example 3 Preparation of Oral Insulin Positioned Release Coated Double Layer Tablets
[0076] The preparation method of this example is the same as that of Example 1, the difference is that the drug-positioned drug release coating material of the back-coated double-layer tablet is prepared from a 95% ethanol solution with a mass ratio of Eudragit L100 and Eudragit S100 of 1:2. For a solution with a mass concentration of 10%, a series of tablets with different localized drug-release coating thicknesses of 50, 90, 110, 140, and 160 μm were also prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com