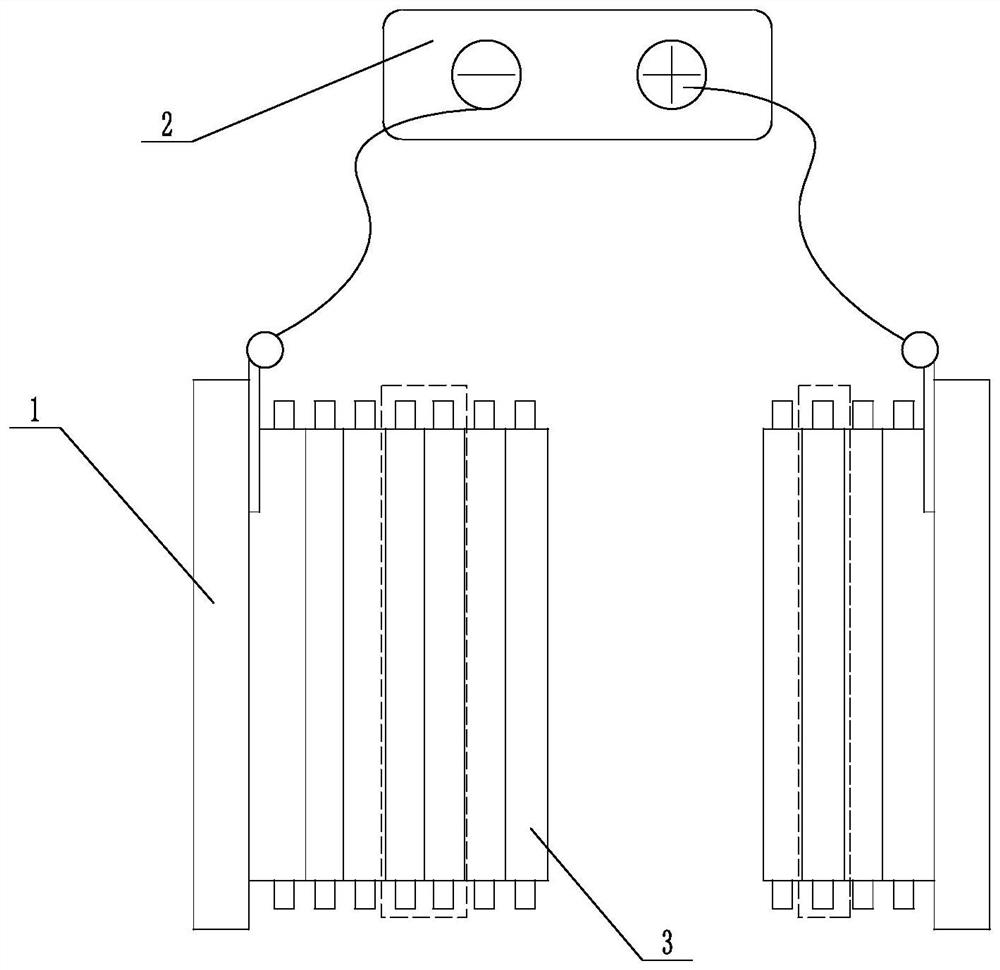
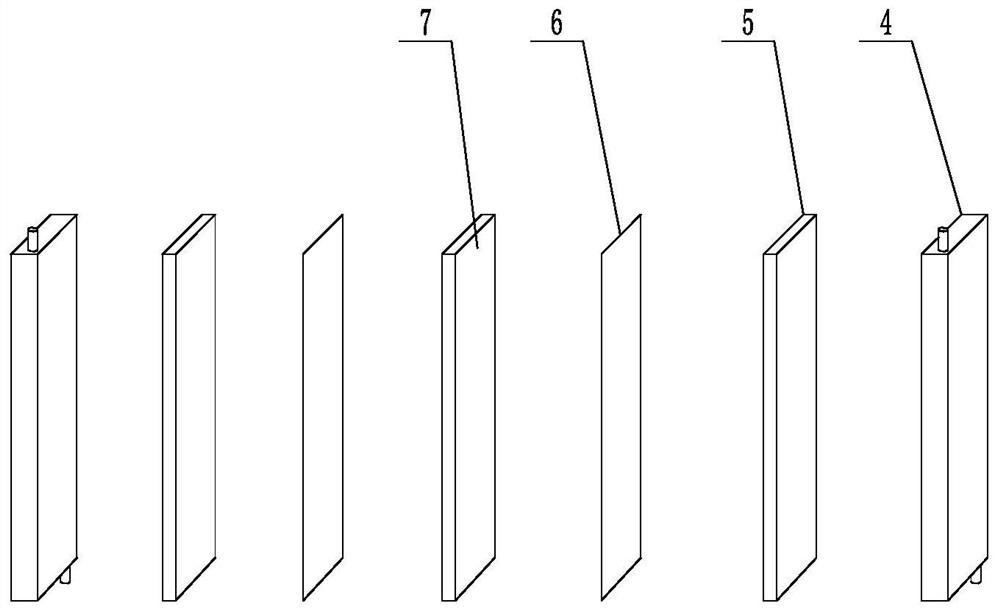
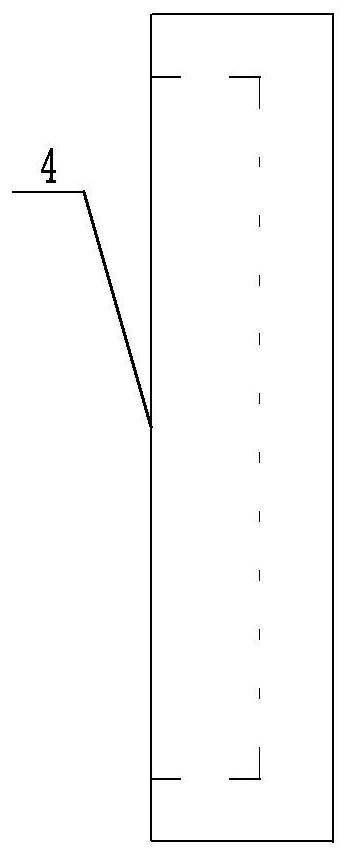
Chlor-alkali membrane electrolyzer with porous conductive plate
A conductive plate and membrane electrolysis technology, applied in the direction of electrolysis process, electrolysis components, electrodes, etc., can solve the problems such as the inability to achieve the increase of cell voltage, achieve the effect of reducing the mechanical performance requirements, eliminating the increase of cell voltage, and high electrolysis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Table 1 Summary table of electrolysis experiment results of different materials
[0028]
[0029] Here, the energy consumption is represented by the cell voltage, and the current density of each batch of experiments is 6kA / m 2 , as can be seen from Table 1, the energy consumption of carbon felt, sintered copper plate and nickel foam is higher than that of ordinary experimental tank. High, so continuous optimization is required.
Embodiment 2
[0031] The device of this embodiment is the same as that of Example 1. The experimental process is based on Example 1, and the optimization experiment is carried out on nickel foams of different thicknesses. The operating current density of each batch of experiments is 6kA / m 2 , other operating conditions and process are all the same as in Example 1, and the experimental results are shown in Table 2.
[0032] Table 2 Summary of electrolytic experiment results with different thicknesses of nickel foam
[0033]
[0034] Here, the energy consumption is also characterized by cell voltage. As can be seen from Table 3, the experimental effect of the nickel foam with a thickness of 0.2mm is better than that of nickel foam with 0.5mm and 1.0mm. From the data of this embodiment, it can be determined that the smaller the nickel foam with thickness The better the effect, but still need to continue to optimize.
Embodiment 3
[0036] The device of this embodiment is the same as that of Example 2. This experimental process is based on Example 2, and whether there is an active coating on the cathode filling material is tested, and the current density of each batch of experiments is 6kA / m 2 , other operating conditions and process are all the same as in Example 2, and the experimental results are shown in Table 3.
[0037] Table 3 Summary table of electrolytic experiment results with or without active coating
[0038]
[0039] Here, the cell voltage is also used to represent energy consumption. As can be seen from Table 2, the experimental effect of nickel foam with active coating is better than that of nickel foam without active coating, but the energy consumption of nickel foam with active coating is comparable to that of ordinary experimental tanks. The ratio is still slightly higher and needs to be continuously optimized.
[0040] The above results prove the novel chlor-alkali membrane electrol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com