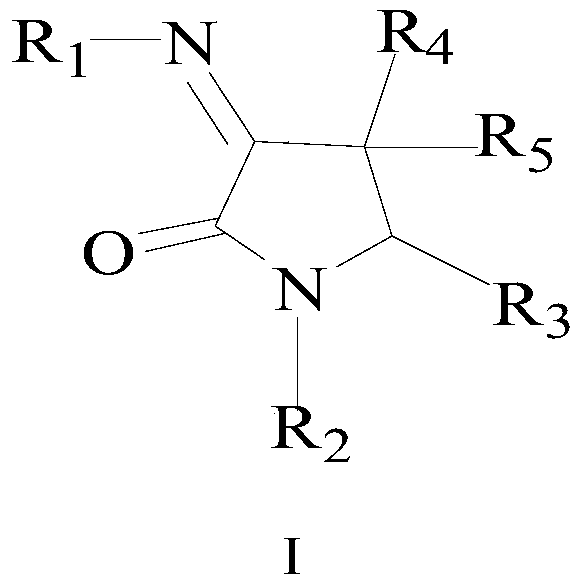
Medicine for treating influenza virus infection
An influenza virus infection, influenza virus technology, applied in the direction of antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、293
[0123] Preparation of embodiment 1, 293T-IAV-Luc cells
[0124] 1. Construction of pREP4-IAV-Luc plasmid
[0125] 1.1. Artificially synthesized DNA fragment shown in sequence 1 SEQ ID NO.: 1 in the sequence listing (see patent CN106562957A). Sequence 1 consists of a total of 1748 nucleotides, the 14th-58th position is recorded as fragment 1, the 59th-1711th position is the firefly luciferase coding gene (reporter gene), the 1712-1734th position is recorded as fragment 2, and the two ends It is the recognition site sequence of BsmB I. Wherein, both fragment 1 and fragment 2 are promoters of influenza virus NP protein, and in the presence of influenza virus, the promoter located on the fusion plasmid can be activated, so that firefly luciferase can be expressed.
[0126] 1.2. Use the restriction endonuclease BsmBI to digest the DNA fragment shown in the sequence 1 SEQ ID NO.: 1 in the sequence list, recover the digested fragment and connect it with the large backbone fragment ...
Embodiment 2
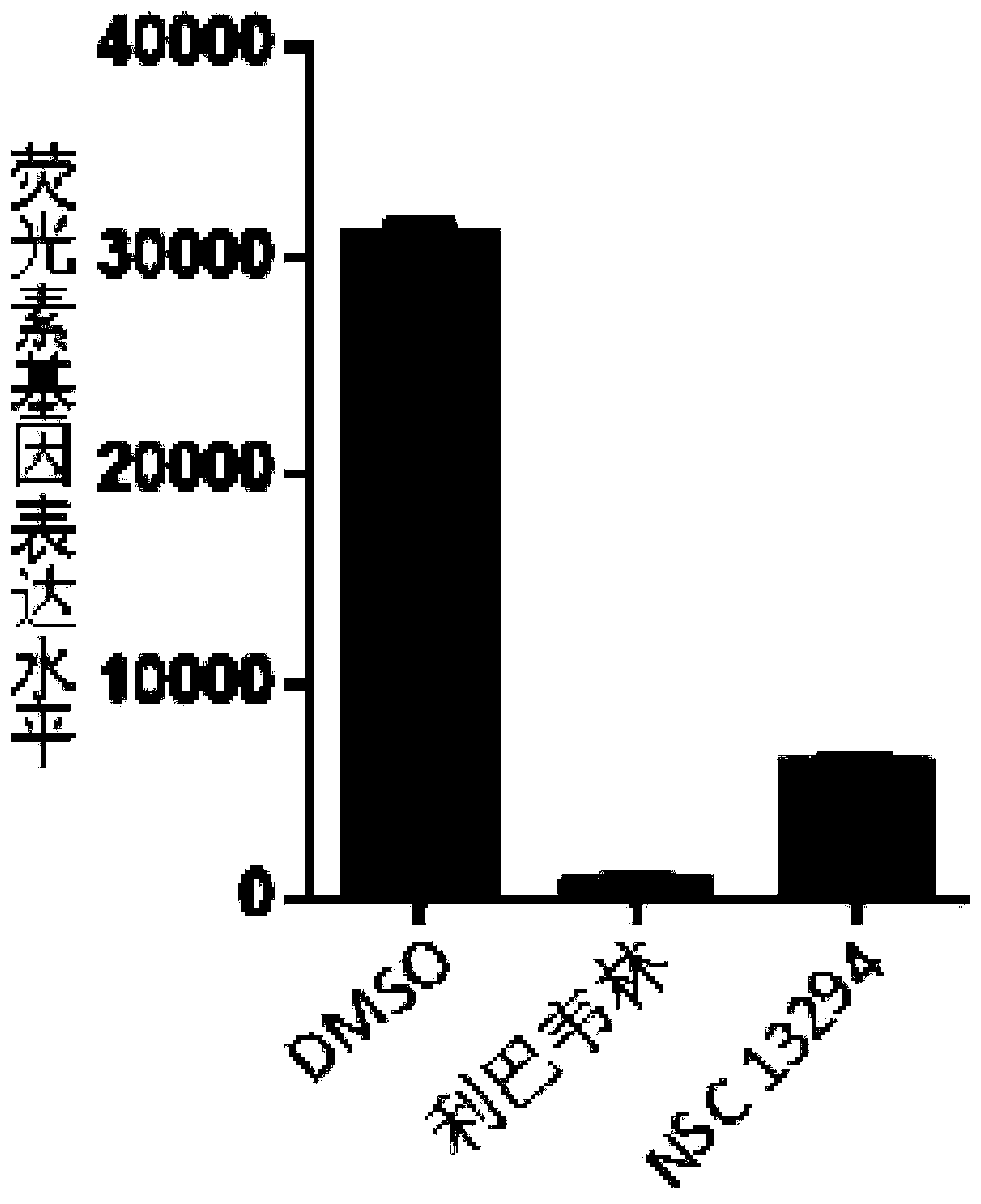
[0129] Example 2, compound NSC 13294 inhibits the replication of H1N1 subtype influenza virus
[0130] 1. Mix A / WSN / 33 strain, DMEM medium and compound NSC 13294 to obtain a mixed solution. The mixture contained 0.5 MOI virus and 50 μmol / L compound NSC 13294.
[0131] 2. Spread the 293T-IAV-Luc cells prepared in Example 1 evenly on a 96-well plate (20,000 cells per well), culture at 37° C. for 12 hours, discard the supernatant, and wash the cells in the well with PBS buffer solution. cell.
[0132] 3. After completing step 2, take the 96-well plate, add the mixed solution obtained in step 1 (MOI=0.5), incubate at 37° C. for 1 hour, and discard the supernatant.
[0133] 4. After completing step 3, take the 96-well plate, add DMEM medium containing 10% (volume ratio) fetal bovine serum and 50 μmol / L compound NSC 13294, and culture at 37° C. for 12 hours, discard the supernatant, and use The cells in the wells were washed with PBS buffer.
[0134] 5. After completing step 4, ...
Embodiment 3
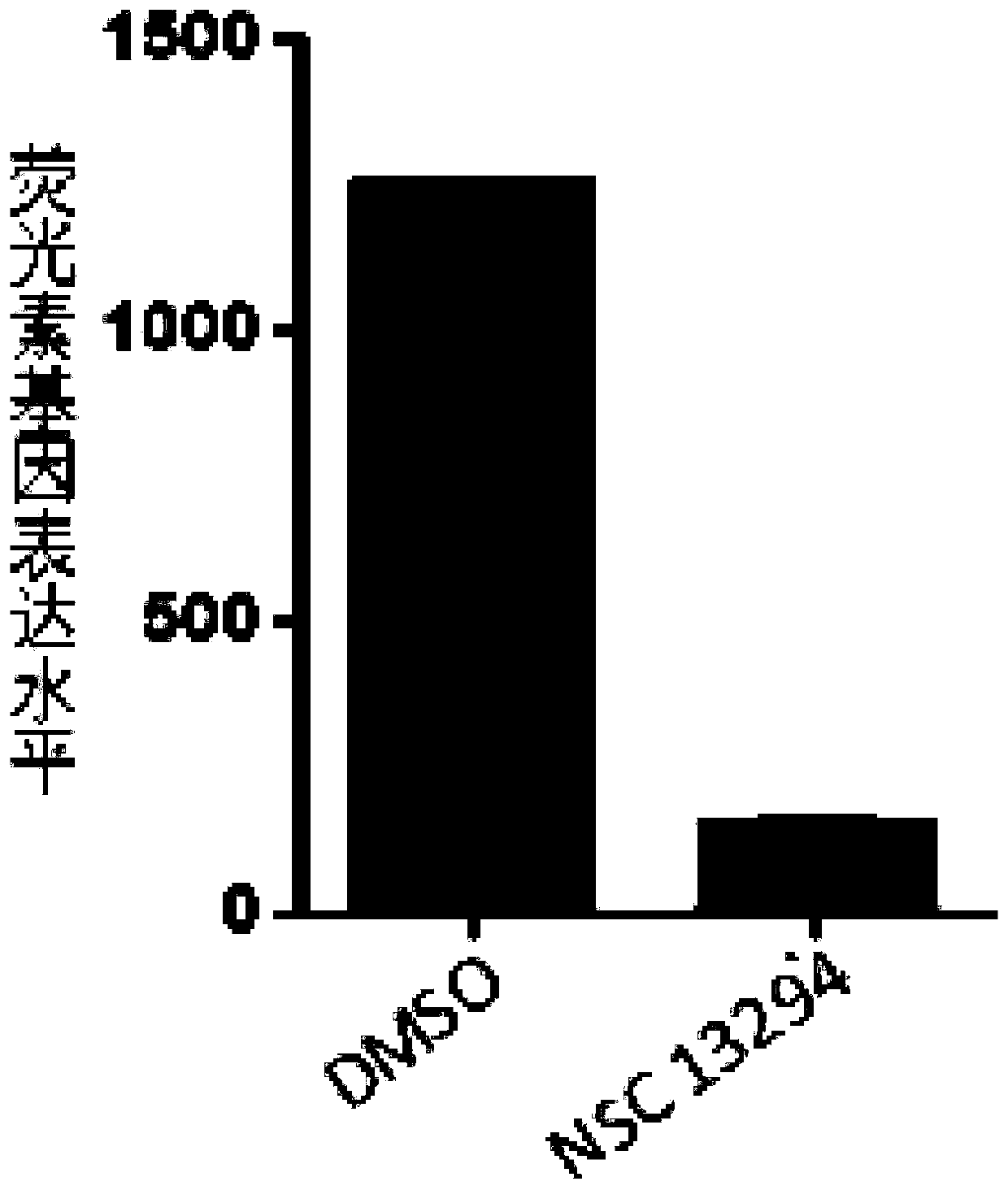
[0142] Embodiment 3, compound NSC 13294 suppresses the activity of HA pseudovirus
[0143] 3.1 Preparation of HA pseudovirus
[0144] 293T cells were inoculated into cell culture dishes, and after reaching 80% density, they were co-transfected with 6 μg pNLLucE-R-HIV-Luc plasmid, 6 μg pEWSN-HA plasmid and 6 μg pCAGGS-NA plasmid with the help of transfection reagent PEI, and replaced with 10 % (volume fraction) DMEM complete medium of fetal bovine serum, after 48 hours, transfer the entire culture system to a 15ml centrifuge tube, blow off the cells, freeze and thaw once, filter with a 0.22 μm filter, and collect the filtrate, which is the HA pseudovirus The virus solution was stored at -80°C.
[0145] 3.2. Compound NSC 13294 inhibits the activity of HA pseudovirus
[0146] 1. Spread 293T cells evenly on a 24-well plate (160,000 cells per well), culture at 37°C for 15 hours, and discard the supernatant.
[0147] 2. After completing step 1, take the 24-well plate, add 100 μL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com