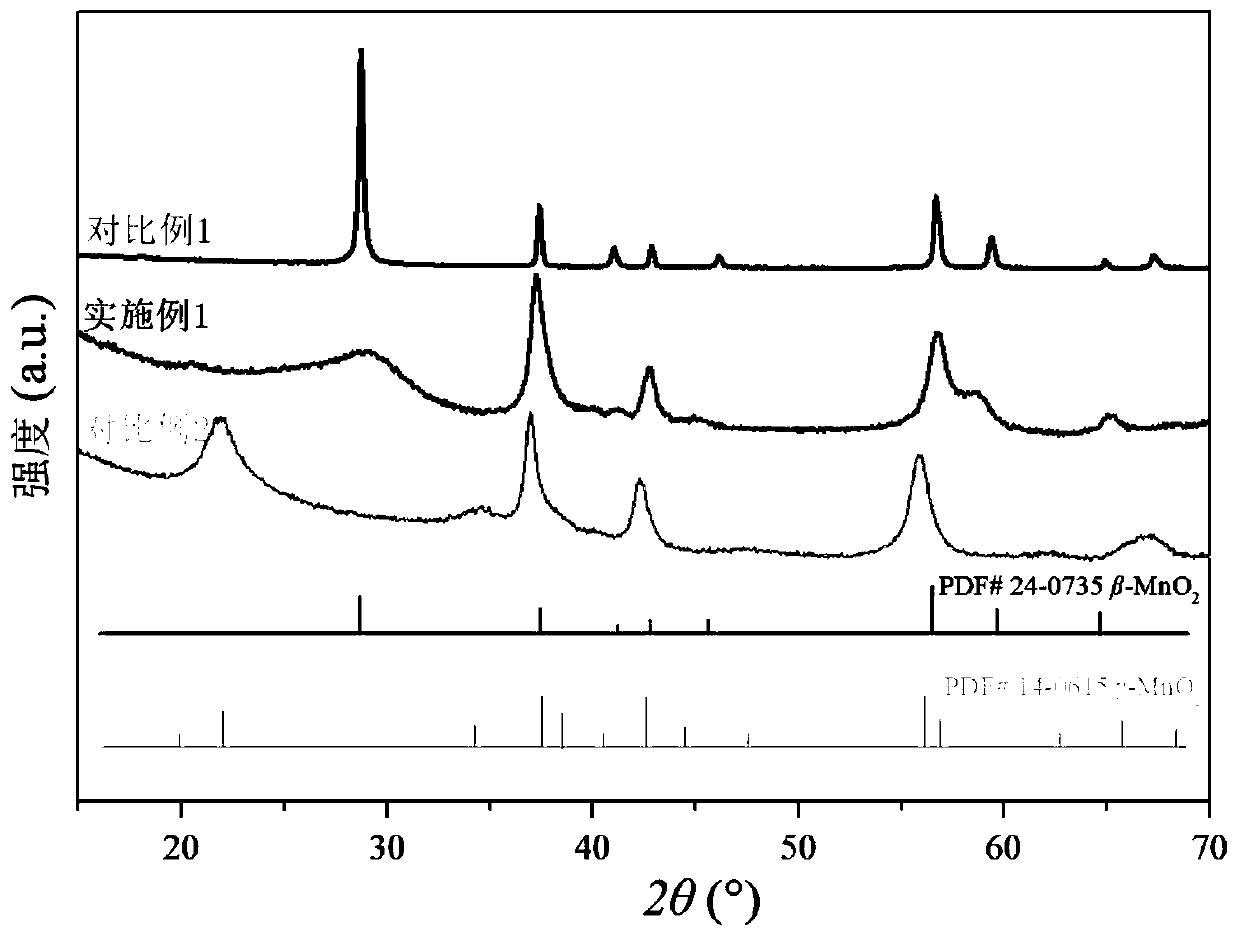
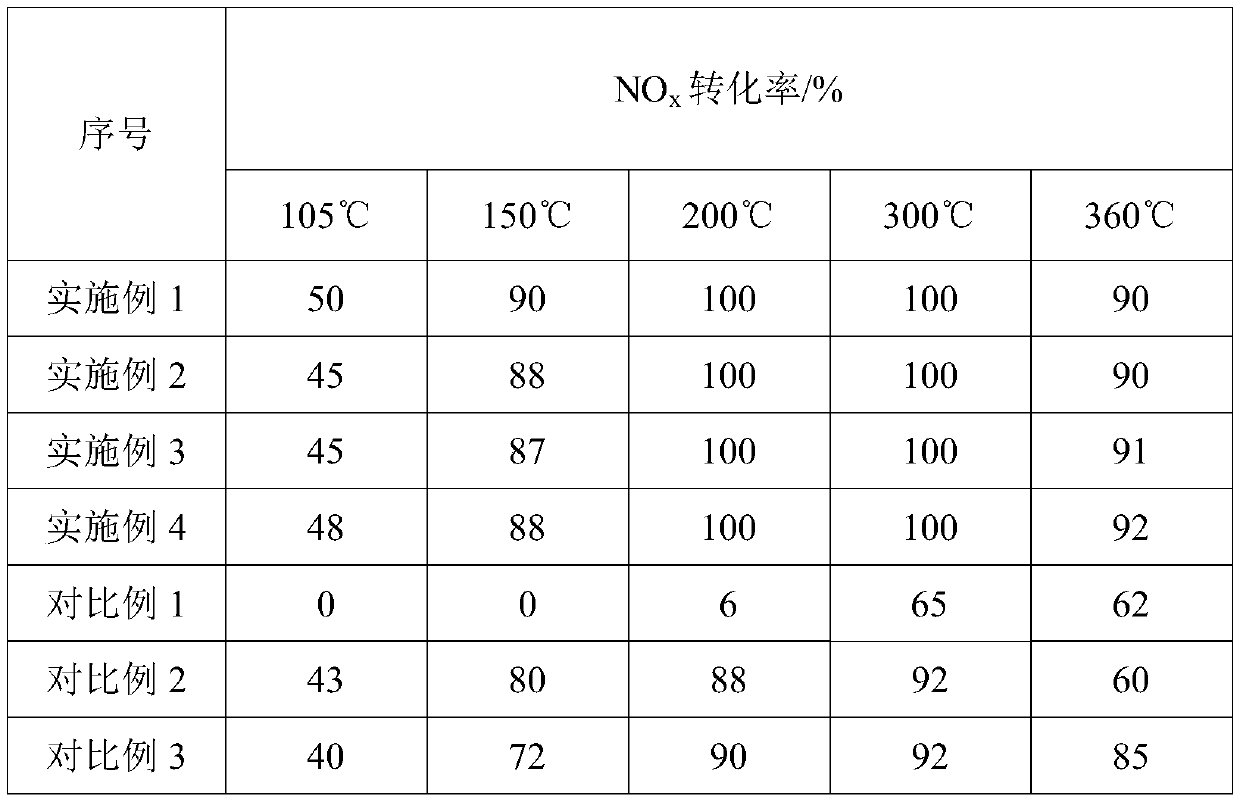
Beta-MnO2 nanosheet catalyst, preparation method and applications thereof
A nanosheet and catalyst technology, applied in the field of β-MnO2 nanosheet catalyst and its preparation, can solve the problems of low denitration activity and the like, and achieve the effects of improving reaction rate, convenient control and good low temperature denitration activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A β-MnO 2 The preparation method of nano sheet catalyst, comprises the steps:
[0034] S1. Manganese sulfate monohydrate (MnSO 4 ·H 2 O) Dissolve completely in pure aqueous solution, then add 40wt.% sodium permanganate (NaMnO 4 ) solution, the molar ratio of manganese sulfate to sodium permanganate is 3:2, after 5 minutes of ultrasonic vibration, stand at 25°C for 24h, suction filter, wash until the filtrate is transparent and dry, and obtain amorphous manganese oxide;
[0035] S2. Disperse the dried amorphous manganese oxide in step S1 in pure aqueous solution, then react at 80°C for 24h, then suction filter, wash, and dry to obtain γ-MnO 2 ;
[0036] S3. the γ-MnO in step S2 2 After calcination at 350 °C for 3 h, β-MnO 2 nanosheet catalysts.
Embodiment 2
[0038] A β-MnO 2 The preparation method of nano sheet catalyst, comprises the steps:
[0039] S1. Manganese sulfate monohydrate (MnSO 4 ·H 2 O) Dissolve completely in pure aqueous solution, then add 40wt.% sodium permanganate (NaMnO 4 ) solution, the molar ratio of manganese sulfate to sodium permanganate is 4:2, after 5 minutes of ultrasonic vibration, stand at 25°C for 24h, suction filter, wash until the filtrate is transparent and dry, and obtain amorphous manganese oxide;
[0040] S2. Disperse the dried amorphous manganese oxide obtained in step S1 in a pure aqueous solution, then react at 80°C for 24h, then suction filter, wash, and dry to obtain γ-MnO 2 ;
[0041] S3. The γ-MnO prepared in step S2 2 β-MnO was obtained after calcination at 350 °C for 3 h 2 nanosheet catalysts.
Embodiment 3
[0043] A β-MnO 2 The preparation method of nano sheet catalyst, comprises the steps:
[0044] S1. Manganese sulfate monohydrate (MnSO 4 ·H 2 O) Dissolve completely in pure aqueous solution, then add 40wt.% sodium permanganate (NaMnO 4) solution, the mol ratio of manganese sulfate to sodium permanganate is 3:2. After ultrasonic vibration for 5 minutes, stand at 25°C for 12 hours, suction filter, wash until the filtrate is transparent and dry, and obtain amorphous manganese oxide;
[0045] S2. Disperse the dried amorphous manganese oxide obtained in step S1 in a pure aqueous solution, then react at 80°C for 8h, then suction filter, wash, and dry to obtain γ-MnO 2 ;
[0046] S3. The γ-MnO prepared in step S2 2 β-MnO was obtained after calcination at 350 °C for 3 h 2 nanosheet catalysts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com