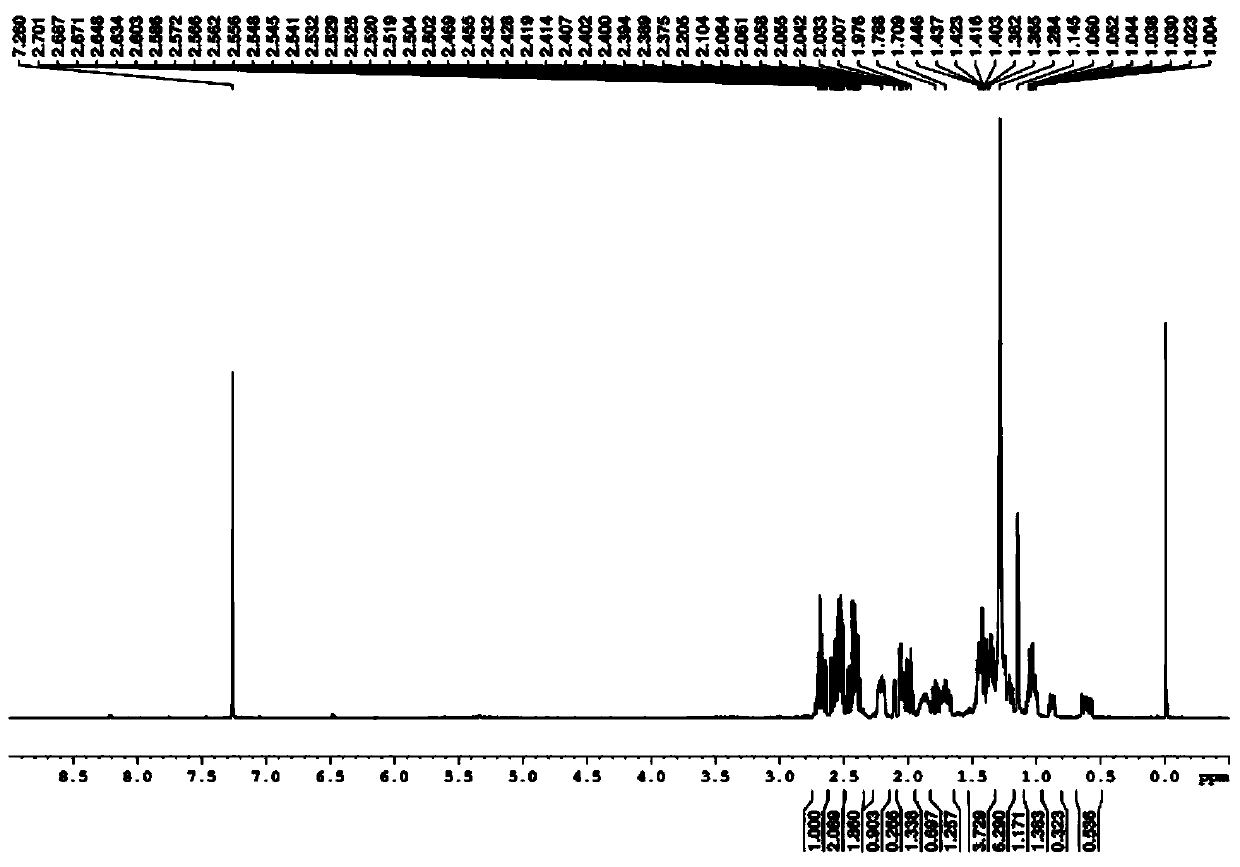
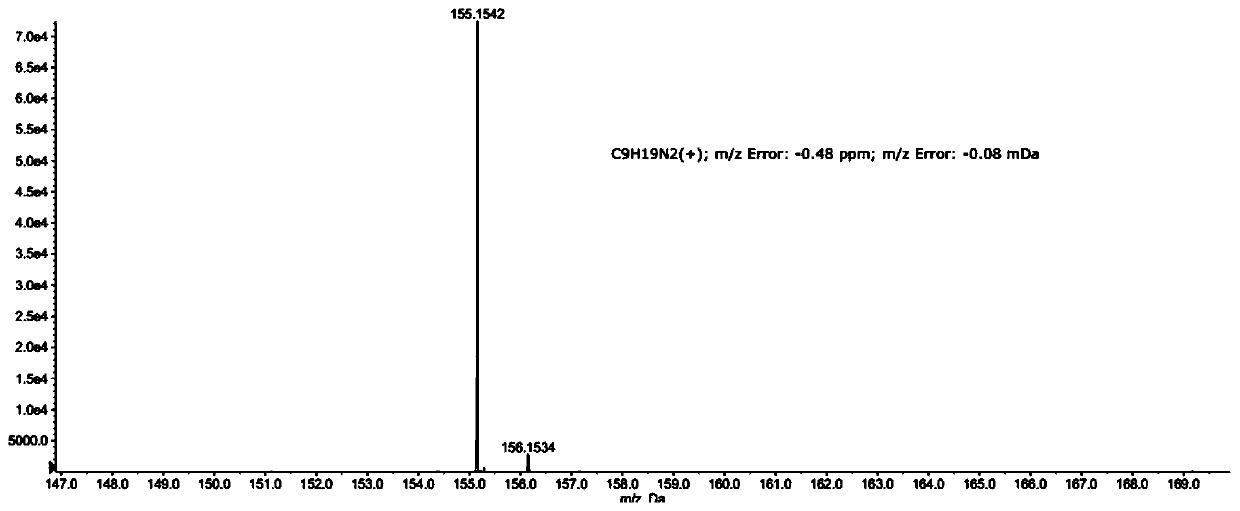
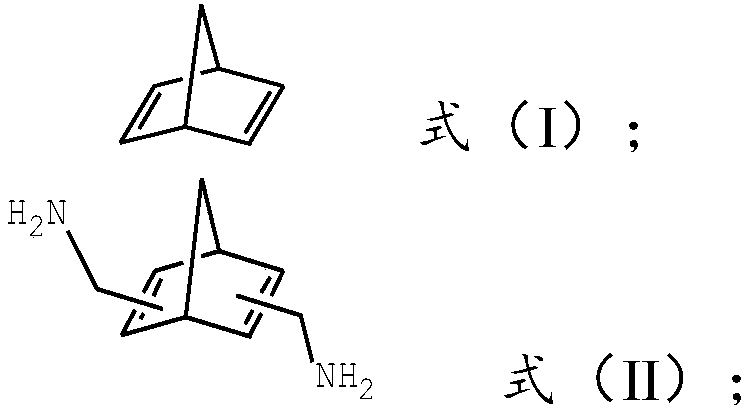
Preparation method of norbornane dimethyl amine
A technology for bornane dimethylamine and compounds is applied in the field of preparation of norbornane dimethylamine, and can solve the problems of complicated methods, complicated operations, complicated post-processing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of norbornane dimethylamine, comprises the following steps:
[0027] a) The compound of the structure shown in formula (I) is mixed with urotropine, acid and the first solvent, and the first reaction is carried out to obtain the first reaction mixture; then the above-mentioned first reaction mixture is sequentially neutralized, the second Once filtered, the filtrate obtained is purified for the first time to obtain a compound of structure shown in formula (II);
[0028]
[0029]
[0030] b) Mix the structural compound shown in formula (II) obtained in step a) with hydrogen, a catalyst, and a second solvent, and perform a second reaction to obtain a second reaction mixture; then filter the above-mentioned second reaction mixture for the second time , The obtained filtrate was purified for the second time to obtain norbornane dimethylamine.
[0031] In the present invention, firstly, the compound of the structure ...
Embodiment 1
[0052] (1) Weigh 9.21g of norbornadiene into a 500mL single-necked flask, add 9.81g of urotropine, 64.47g of sulfuric acid, and 138.15g of acetone in sequence, and react for 15h in an oil bath at T=70°C. The sodium carbonate solution system is adjusted to neutrality, then filter, obtain the filtrate that contains the structure compound shown in formula (II);
[0053]
[0054] Then in the separatory funnel, the filtrate is layered to obtain the organic phase, and then the organic phase is rotary evaporated to obtain 13.82g norbornene dimethylamine; the above steps are repeated to obtain more target products, and a total of 27.63g norbornene is obtained ethylene dimethylamine.
[0055] (2) Weigh 15.02g of norbornene dimethylamine obtained in step (1) in a 500mL autoclave, add 1.05g of palladium carbon and 376.25g of toluene successively therein, then pressurize to 0.7MPa with hydrogen and Keep this pressure in the middle, raise the temperature to 95°C and react for 5h, filte...
Embodiment 2
[0059] (1) Weigh 9.21g of norbornadiene into a 250mL single-necked flask, add 5.61g of urotropine, 36.84g of acetic acid, and 92.1g of acetonitrile in sequence, and react for 10h in an oil bath at T=50°C. After the reaction is completed, use saturated The sodium carbonate solution system is adjusted to neutrality, then filter, obtain the filtrate that contains the structure compound shown in formula (II);
[0060]
[0061] Then in the separatory funnel, the filtrate is layered to obtain the organic phase, and then the organic phase is rotary evaporated to obtain 14.27g norbornene dimethylamine; the above steps are repeated to obtain more target products, and a total of 28.55g norbornene is obtained ethylene dimethylamine.
[0062] (2) Weigh 15.02g of norbornene dimethylamine obtained in step (1) in a 500mL autoclave, add 1.05g of Raney nickel and 376.25g of methanol successively therein, then pressurize to 0.9MPa with hydrogen and react During the process, the pressure was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com