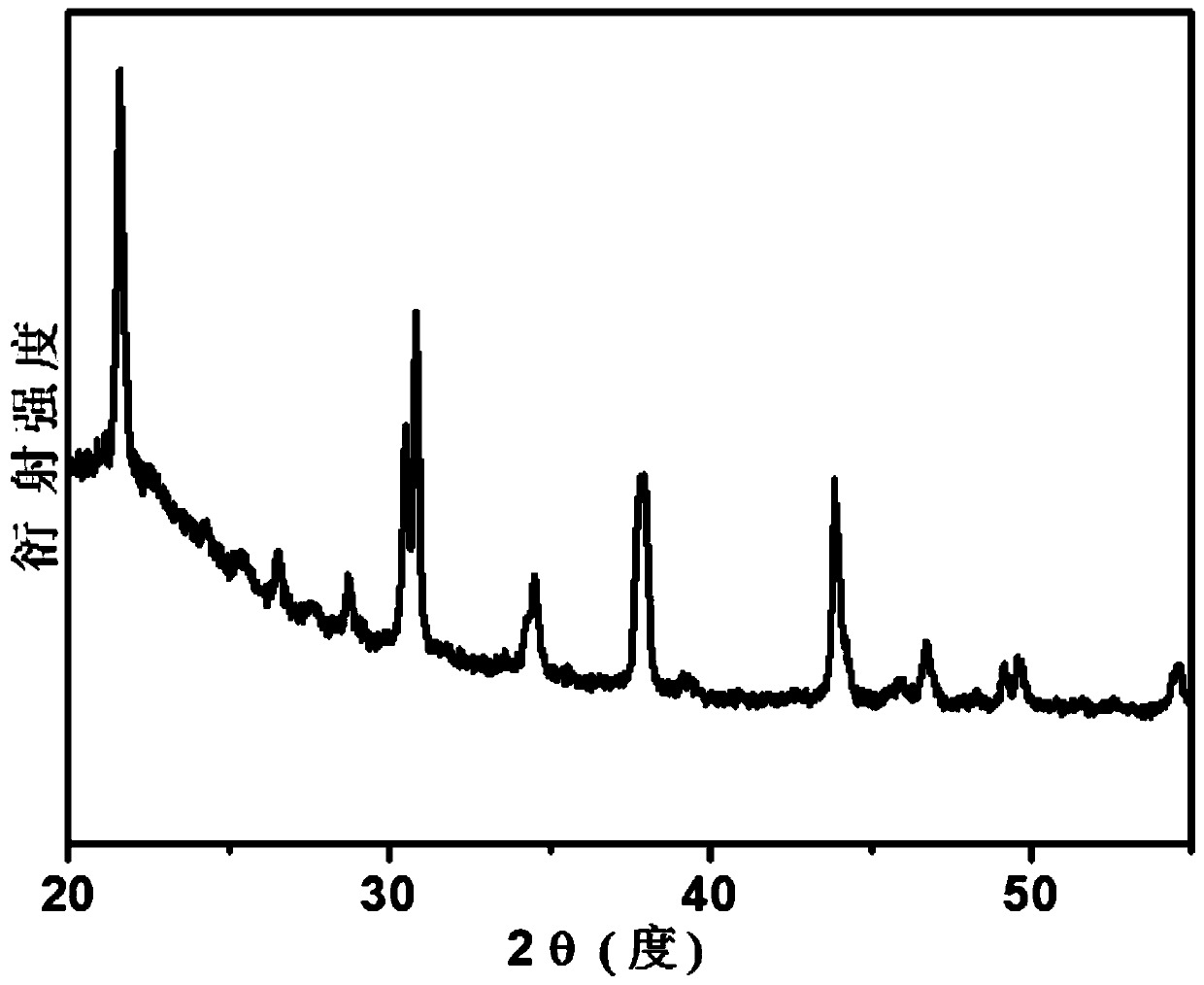
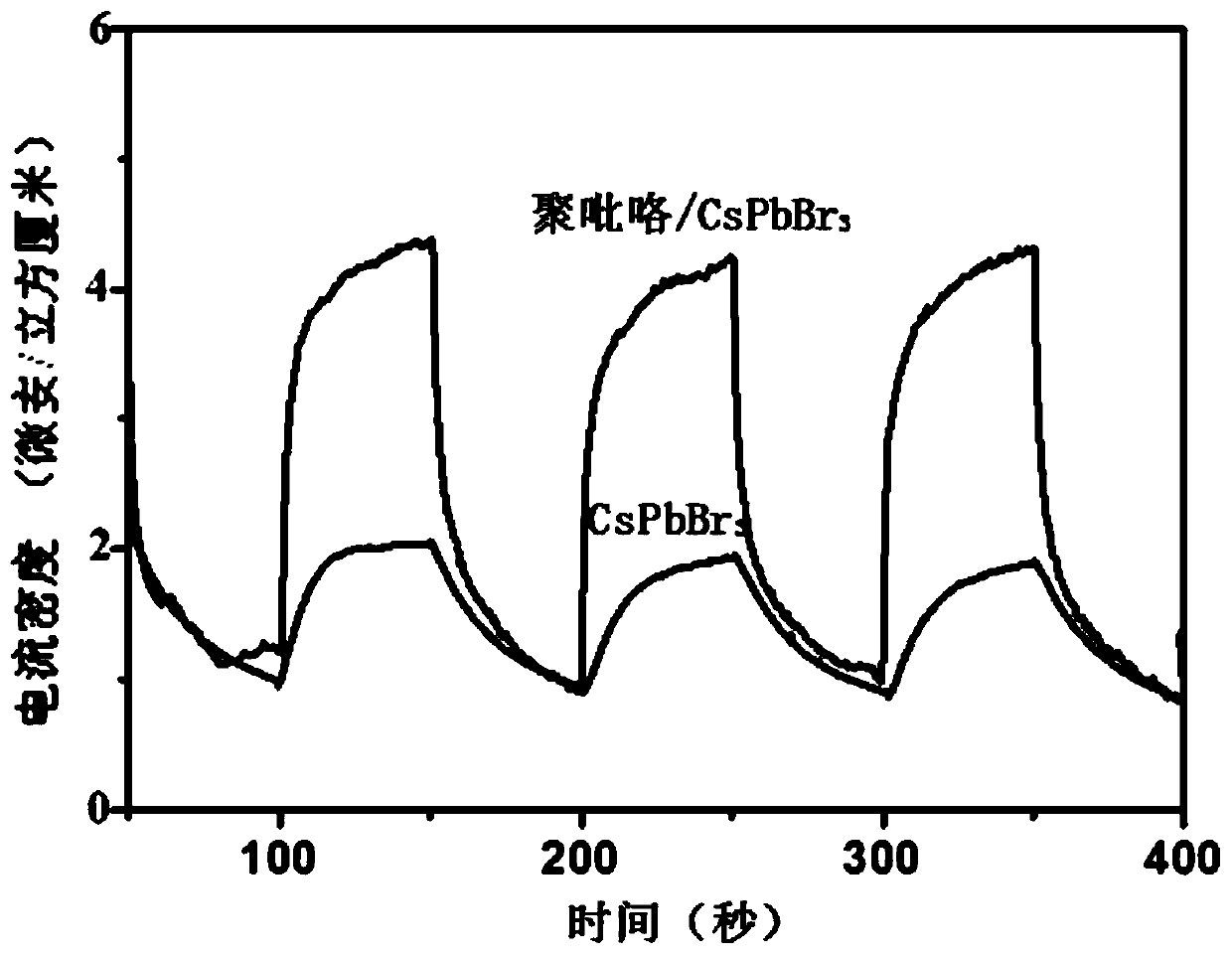
Method for improving stability of all-inorganic perovskite quantum dots CsPbBr3
A technology of quantum dots and inorganic calcium, applied in the field of materials science, can solve problems such as constraints and instability, and achieve the effects of improving stability, improving electrical conductivity and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] An improved all-inorganic perovskite quantum dot CsPbBr 3 The method of stability includes the following steps:
[0029] Step 1: The PbBr 2 Mix with octadecene in a molar ratio of 0.007:1 to obtain solution I;
[0030] Step 2: in an inert environment, add oleylamine and oleic acid in a volume ratio of 1:1 to the solution I obtained in step 1 to obtain solution II;
[0031] Step 3: Raise the reaction temperature of solution II to 160°C, then quickly add the cesium precursor solution to solution II, keep this temperature for 5 seconds, then cool down with an ice-water bath, centrifuge and precipitate, and then disperse the quantum dots in the solution II. 20mL of toluene to obtain CsPbBr 3 quantum dot solution;
[0032] Step 4: Add 35 mg of pyrrole and 40 mg of benzoquinone to the CsPbBr obtained in step 3 3 into the quantum dot solution, and then add 20 mL of chloroform to obtain a reaction system;
[0033] Step 5: use a 500W xenon lamp as the light source, install...
Embodiment 2
[0040] The only difference between this example and Example 1 is that the amount of pyrrole added is 30 mg, and the rest of the contents are exactly the same as those described in Example 1. Known through detection and analysis: the polypyrrole obtained in this example
[0041] / CsPbBr 3 The photocurrent density of the sample under the same conditions is pure CsPbBr 3 1.5 times that of quantum dots, which is lower than the photocurrent density of the material obtained in Example 1.
Embodiment 3
[0043] The only difference between this example and Example 1 is that the amount of benzoquinone added is 35 mg, and the rest of the content is exactly the same as that described in Example 1. Known through detecting and analyzing: the polypyrrole obtained in the present embodiment
[0044] / CsPbBr 3 The photocurrent density of the sample under the same conditions is that of pure CsPbBr 3 1.9 times that of quantum dots, and the photocurrent density of the material obtained in Example 1 has decreased.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com