Oral-taking rehydration salt, preparation method and applications thereof
A technology of rehydration salt and animal bifidobacteria, which is applied in the field of medicine, can solve the problems of limited treatment of infantile diarrhea, further improvement of diarrhea treatment, and poor defense function of the body, so as to reduce the secretion of water and electrolytes in the intestinal mucosa and significantly treat diarrhea. Effect, the effect of correcting the imbalance of intestinal flora
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
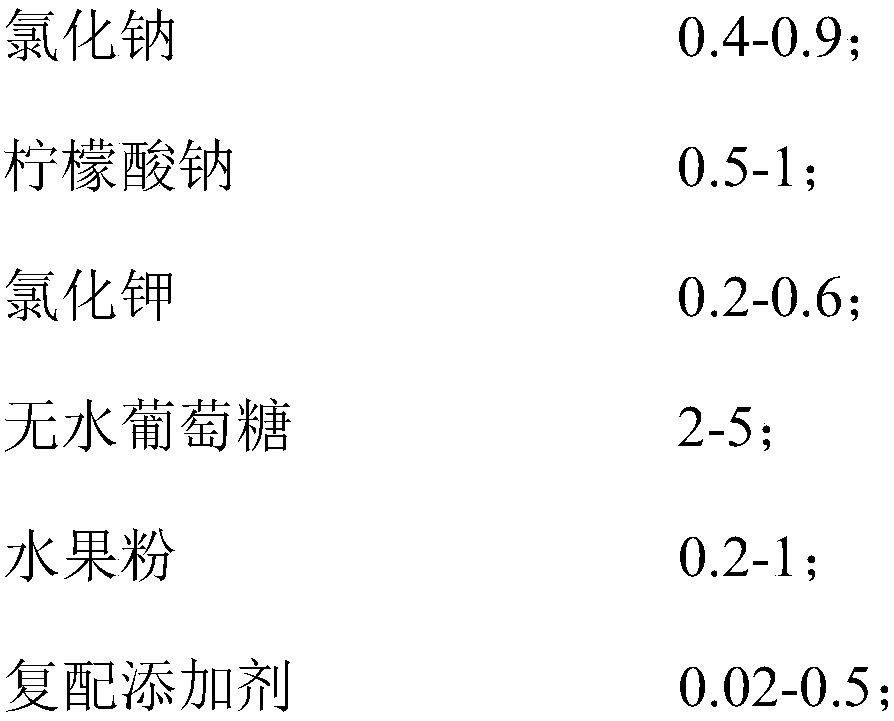
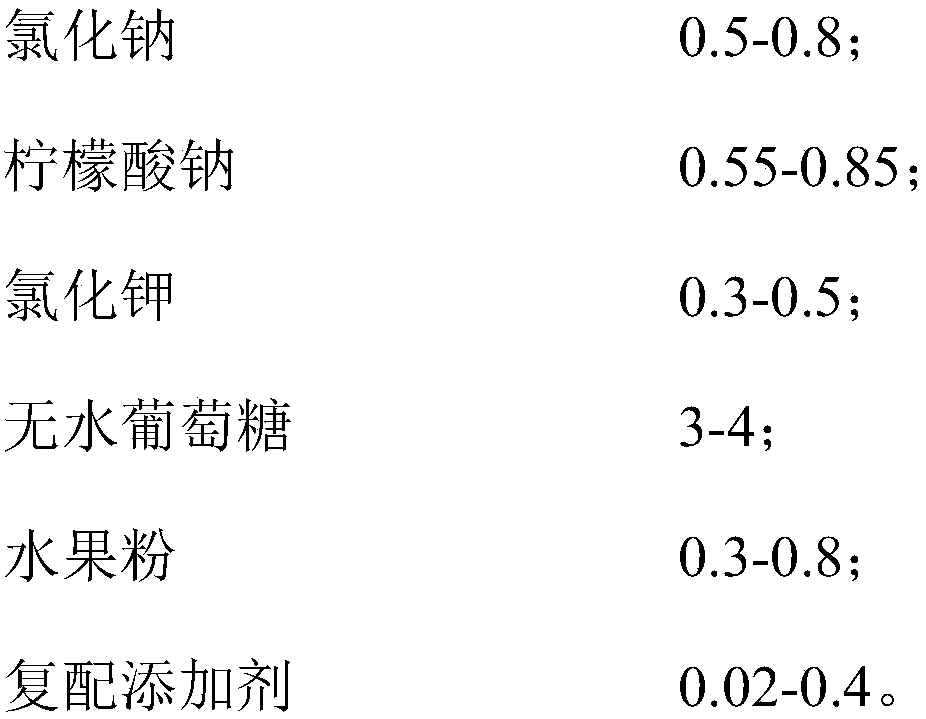
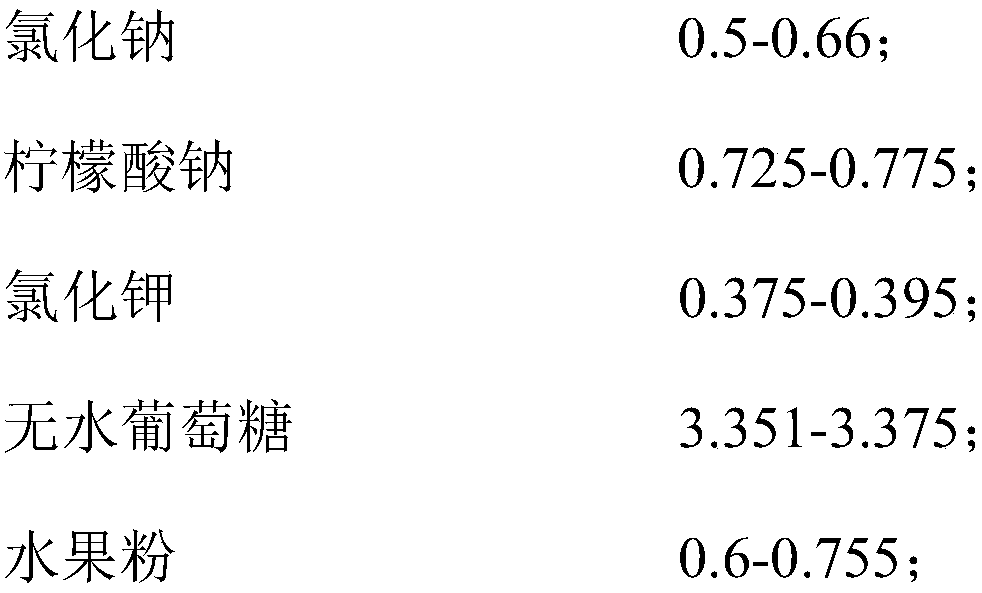
[0035] Prescription: 0.66 parts by weight of sodium chloride, 0.725 parts by weight of sodium citrate, 0.375 parts by weight of potassium chloride, 3.375 parts by weight of anhydrous glucose, 0.01 parts by weight of zinc gluconate, 0.1 parts by weight of animal bifidobacteria Bb-12 (the content of viable bacteria is about 30 billion CFU / g), 0.755 parts by weight of strawberry fruit powder.
[0036] Preparation:
[0037] (1) Dry the prescription amount of anhydrous dextrose and strawberry fruit powder at a temperature not exceeding 60°C for 2-3 hours, and control the moisture content within 3%; dried anhydrous dextrose and fruit Powder through a 60 mesh sieve;
[0038] (2) Crush the prescription amount of sodium chloride, potassium chloride and sodium citrate through a 60-mesh sieve;
[0039] (3) Thaw the prescription amount of Bifidobacterium animalis Bb-12 to room temperature in advance, and mix it with zinc gluconate to obtain a compound additive;
[0040] (4) Mix the anhydrous gluc...
Embodiment 2
[0042] Prescription: 0.5 parts by weight of sodium chloride, 0.775 parts by weight of sodium citrate, 0.395 parts by weight of potassium chloride, 3.51 parts by weight of anhydrous glucose, 0.02 parts by weight of zinc gluconate, 0.2 parts by weight of animal bifidobacteria Bb-12 (the number of live bacteria is about 30 billion CFU / g), 0.6 parts by weight of strawberry fruit powder.
[0043] The preparation method of the oral replenishing salt solution of this example is the same as that of Example 1, and the obtained composition is named FX2.
Embodiment 3
[0045] Prescription: 0.8 parts by weight of sodium chloride, 0.575 parts by weight of sodium citrate, 0.315 parts by weight of potassium chloride, 3.95 parts by weight of anhydrous glucose, 0.01 parts by weight of zinc gluconate, 0.04 parts by weight of animal bifidobacteria Bb-12 (the content of viable bacteria is about 30 billion CFU / g), 0.31 parts by weight of strawberry fruit powder.
[0046] The preparation method of the oral replenishing salt solution of this example is the same as that of Example 1, and the obtained composition is named FX3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com