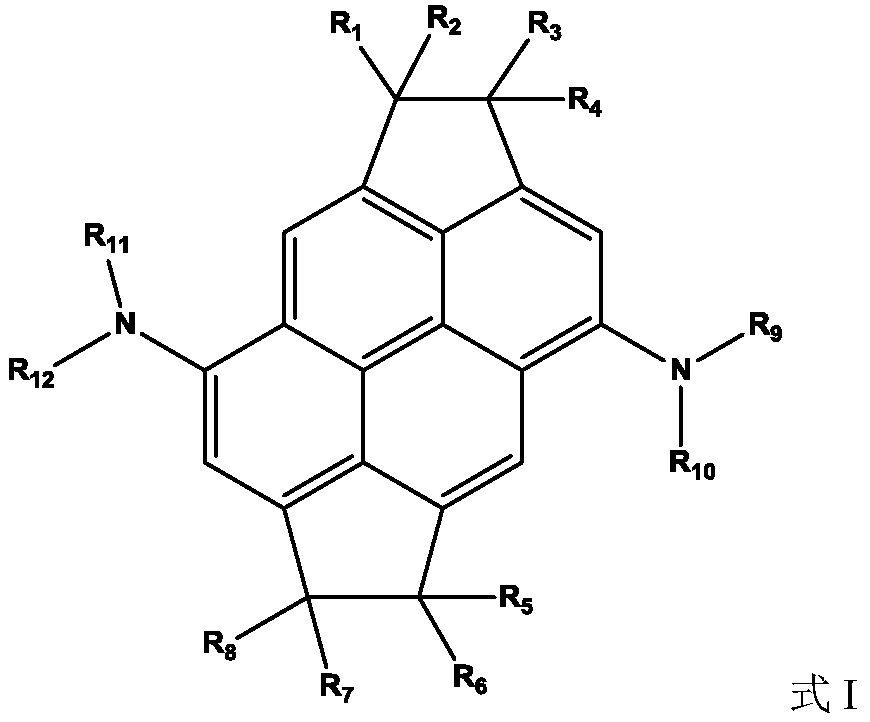
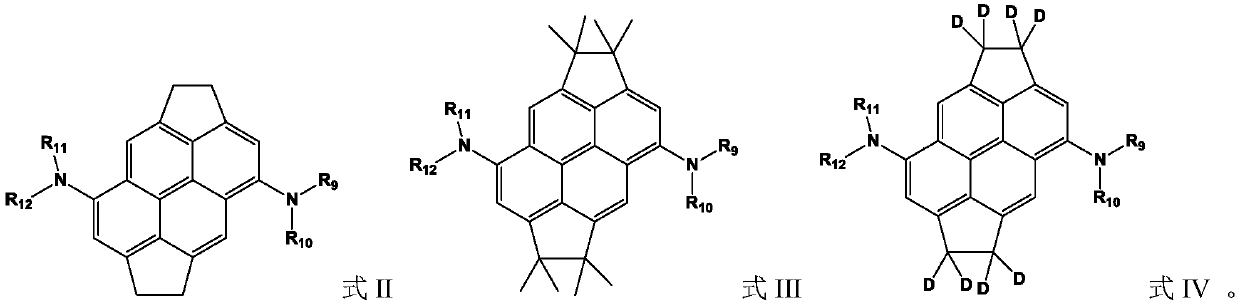
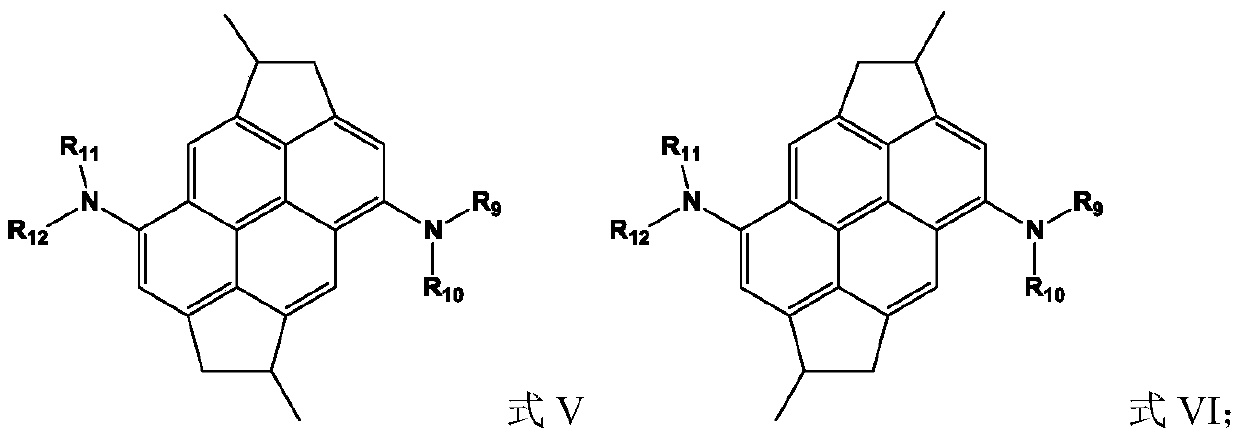
Organic compound containing condensed ring structure and organic electroluminescent device
A technology of organic compounds and fused ring structures, which is applied in the field of organic compounds and organic electroluminescent devices, can solve the problems of low driving voltage service life, narrow emission peak half-peak width, short service life, etc., to increase life and improve overall performance The effect of increasing and decreasing the current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0135] Preparation Example 1: Synthesis of Compound 1:
[0136]
[0137] Synthesis of Compound 1: Add 4.09g (10mmol) of Intermediate B in a 100ml three-necked flask, add 40ml of toluene, stir to dissolve, add 3.38g (20mmol, 2eq.) of diphenylamine, 4.8g (50mmol, 5eq.) of tert-butyl Sodium alkoxide, 0.2mmol tris(dibenzylideneacetone)dipalladium, 0.2mmol tri-tert-butylphosphine, stirred under the protection of nitrogen, heated to reflux, after 4h, TLC detected that the reaction was complete, and the reaction solution was spin-dried under reduced pressure. The solvent was used to obtain a light yellow solid, which was recrystallized twice from toluene to obtain 4.0 g of a light yellow solid (yield 68%).
[0138]1H-NMR (400MHz, CDCl3) (ppm) δ = 3.51 ~ 3.53 (8H, s), 6.96 ~ 6.98 (1H, m), 6.99 ~ 7.01 (2H, m), 7.02 ~ 7.04 (1H, m), 7.05 ~7.07(3H, m), 7.08~7.10(5H, m), 7.20~7.22(3H, m), 7.24~7.25(4H, m), 7.27~7.28(1H, m), 7.81~7.82(2H, s), 7.98-7.99 (2H, s).
preparation example 2
[0139] Preparation Example 2: Synthesis of Compound 7:
[0140]
[0141] Synthesis of Compound 7: The synthesis method was the same as that of Compound 1, and 5.29 g of a light yellow solid was obtained (70% yield).
[0142] 1H-NMR (400MHz, CDCl3) (ppm) δ = 1.17 ~ 1.19 (12H, s), 1.20 ~ 1.22 (12H, s), 2.81 ~ 2.92 (4H, m), 3.53 ~ 3.50 (8H, m), 7.02 ~7.04(3H, m), 7.05~7.07(5H, m), 7.14~7.16(5H, m), 7.17~7.19(3H, m), 7.79~7.81(2H, m), 7.89~7.91(2H, m).
preparation example 3
[0143] Preparation Example 3: Synthesis of Compound 14:
[0144]
[0145] Synthesis of intermediate 14-1: the synthesis method was the same as that of compound 1, and 1.89 g of white solid was obtained (yield 73%).
[0146] Synthesis of Compound 14: The synthesis method was the same as that of Compound 1, and 5.22 g of a light yellow solid was obtained (68% yield).
[0147] 1H-NMR (400MHz, CDCl3) (ppm) δ = 3.50 ~ 3.53 (8H, s), 6.95 ~ 7.02 (2H, m), 7.04 ~ 7.09 (4H, m), 7.19 ~ 7.23 (3H, m), 7.25 ~7.33(3H, m), 7.34~7.41(2H, m), 7.43~7.46(4H, m), 7.50~7.55(2H, m), 7.86~7.89(2H, m), 7.93~7.99(2H, m), 8.01 ~ 8.04 (2H, m), 8.22 ~ 8.19 (2H, m).
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminous efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com