Thermally activated delayed fluorescent material constructed based on nitrogen-containing heteroaromatic ring derivative and carbonyl and application of thermally activated delayed fluorescent material
A technology of thermally activated delay and fluorescent materials, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low oscillator strength, low luminous quantum efficiency, large half-peak width, etc., and achieve easy adjustment and high luminous efficiency , the effect of small half-peak width
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In formula (1) provided by the present invention, when R 1 is tert-butyl, R 2 and R 3 for H, for When, be following formula compound 2, its synthetic route is as follows:
[0023]
[0024] Synthesis of compound 1:
[0025] Tert-butylcarbazole (13.43g, 48mmol), tert-butyl iodobenzene (15.00g, 58mmol), cuprous iodide (0.91g, 4.8mmol), o-phenanthroline (1.73g, 9.6mmol), carbonic acid Potassium (19.87g, 14.4mmol) and 18-crown-6 (1.90g, 7.2mmol) were added to dioxane (60ml), and reacted overnight at 110°C under nitrogen protection. Cool, spin dry, extract with dichloromethane / water, dry the organic phase with anhydrous sodium sulfate, concentrate and then silica gel column chromatography (petroleum ether:dichloromethane=5:1) to obtain 17.91g of white solid, yield 90.68% . 1 H NMR (500MHz, CDCl 3 )δ8.14(d, J=1.7Hz, 2H), 7.57(d, J=8.5Hz, 2H), 7.48–7.42(m, 4H), 7.35(d, J=8.6Hz, 2H), 1.46( s,18H),1.41(s,9H). 13 C NMR (101MHz, CDCl 3 )δ149.91, 142.61, 139.39, 135....
Embodiment 2
[0029] In formula (1) provided by the present invention, when R 1 , R 2 and R 3 for H, respectively When, be following formula compound 5,8,11, its synthetic route is as follows:
[0030]
[0031] Synthesis of compound 3:
[0032] Acridine (4.18g, 20mmol), methyl 2-iodobenzoate (5.76g, 22mmol), cuprous iodide (381mg, 2mmol), copper (1.3mg, 20mmol) and potassium carbonate (3.03g, 22mmol) Add it into dichlorobenzene (30 mL), and react at 190° C. for 24 h under the protection of nitrogen. Cool, filter, wash the solid with dichloromethane, concentrate the filtrate, and perform silica gel column chromatography (petroleum ether:dichloromethane=2:1) to obtain 5.89 g of a light green solid with a yield of 85.8%. 1 H NMR (400MHz, CDCl 3 )δ8.20–8.14(m,1H),7.76(td,J=7.7,1.5Hz,1H),7.63–7.56(m,1H),7.45(dd,J=7.5,1.5Hz,2H),7.34 (d,J=7.8Hz,1H),6.97–6.85(m,4H),6.05(d,J=9.1Hz,2H),3.54(s,3H),1.71(d,J=22.8Hz,6H) . 13C NMR (126MHz, CDCl 3 )δ165.95, 140.70, 140.43, 134.58, 133.4...
Embodiment 3
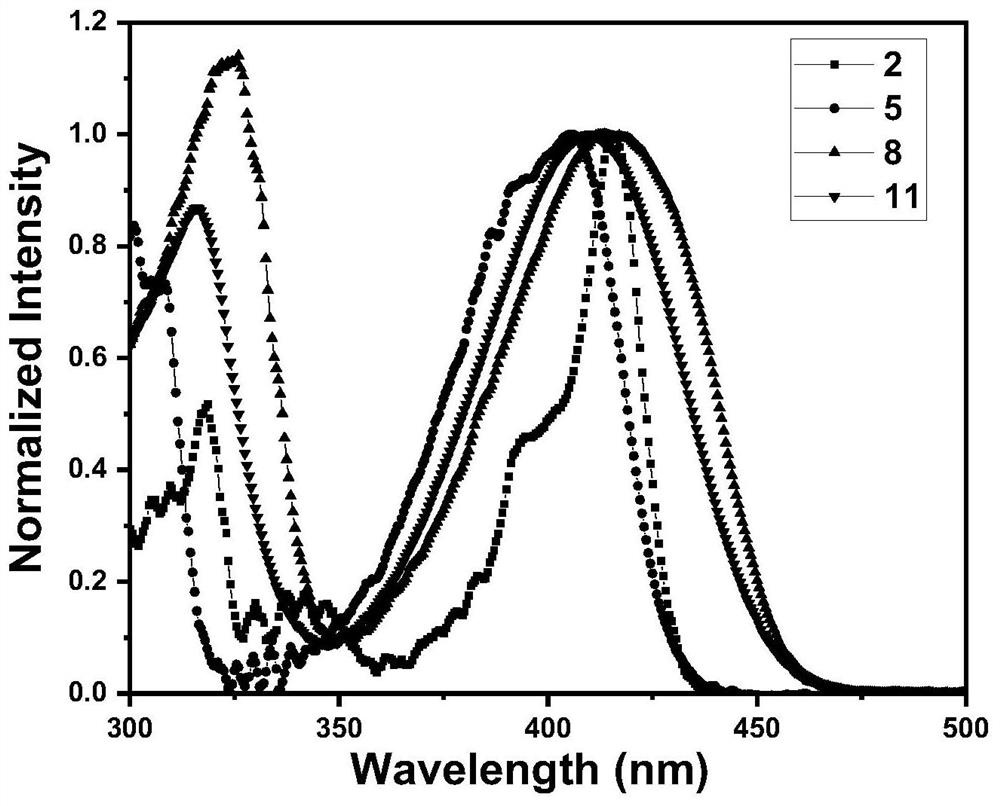
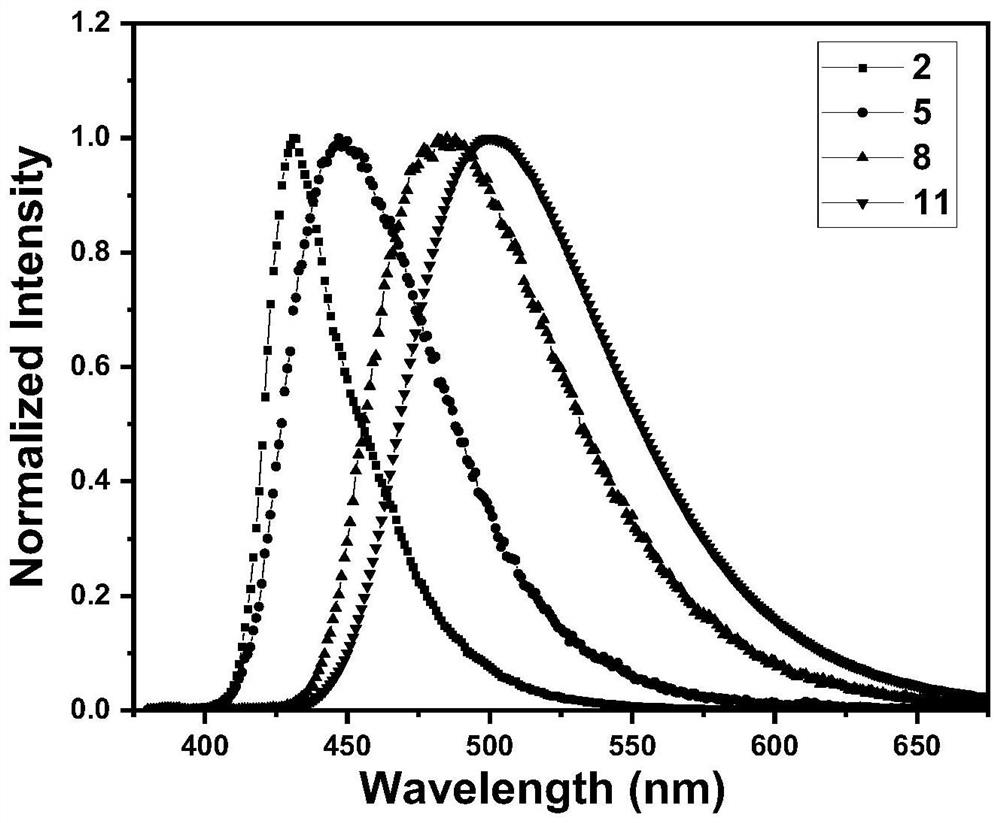
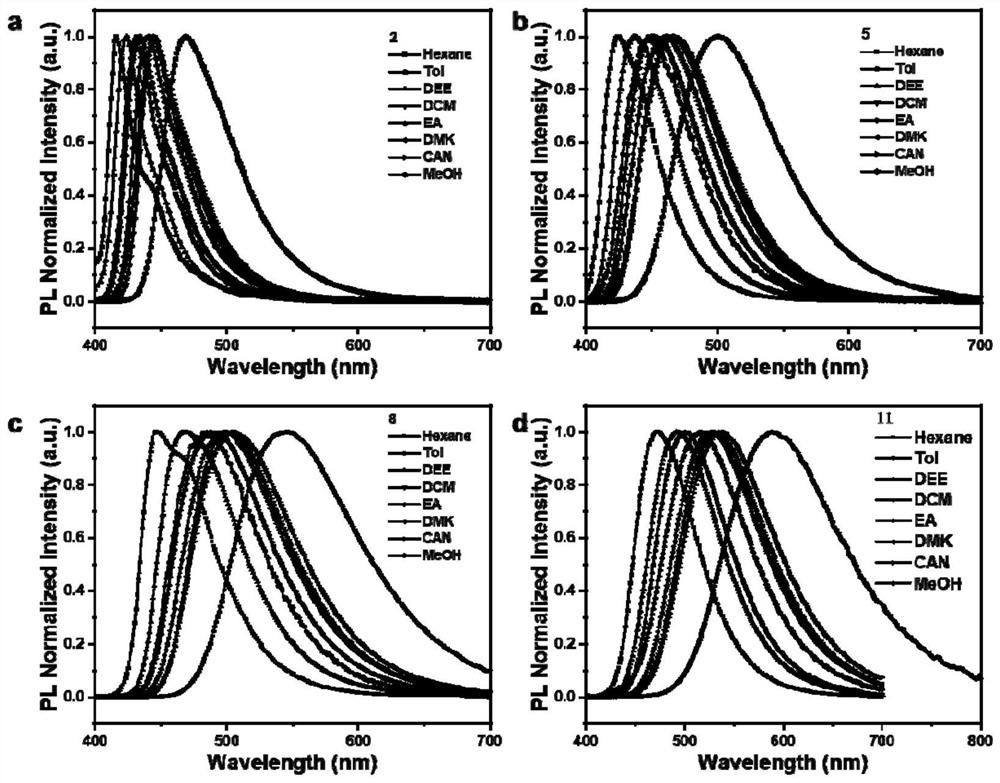
[0052] Compounds 2, 5, 8, and 11 were dissolved in toluene to form 10 -5 M solution, test the UV-Vis absorption spectrum of its solution. Depend on figure 1 It can be seen that there are roughly two absorption peaks in the UV-Vis absorption spectra of these three types of compounds in solution: the absorption peak at the short wavelength (325nm) is mainly attributed to the π-π* transition absorption of the molecule; the long wavelength (400-410nm) The absorption peak of is attributed to the intramolecular charge transfer (ICT) transition from the donor unit to the acceptor unit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com