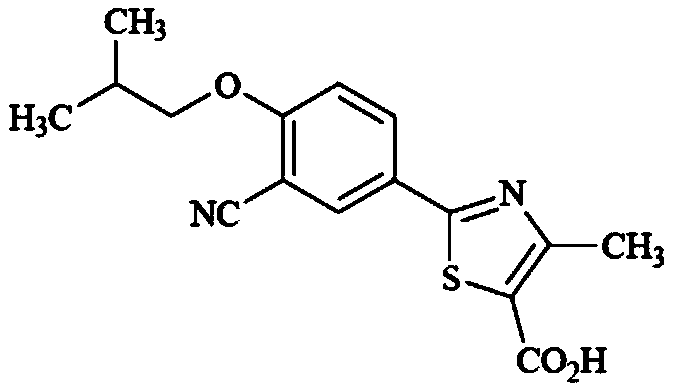
Method for synthesizing febuxostat and intermediate thereof
A reaction mixture, methyl technology, applied in the field of medicine, can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
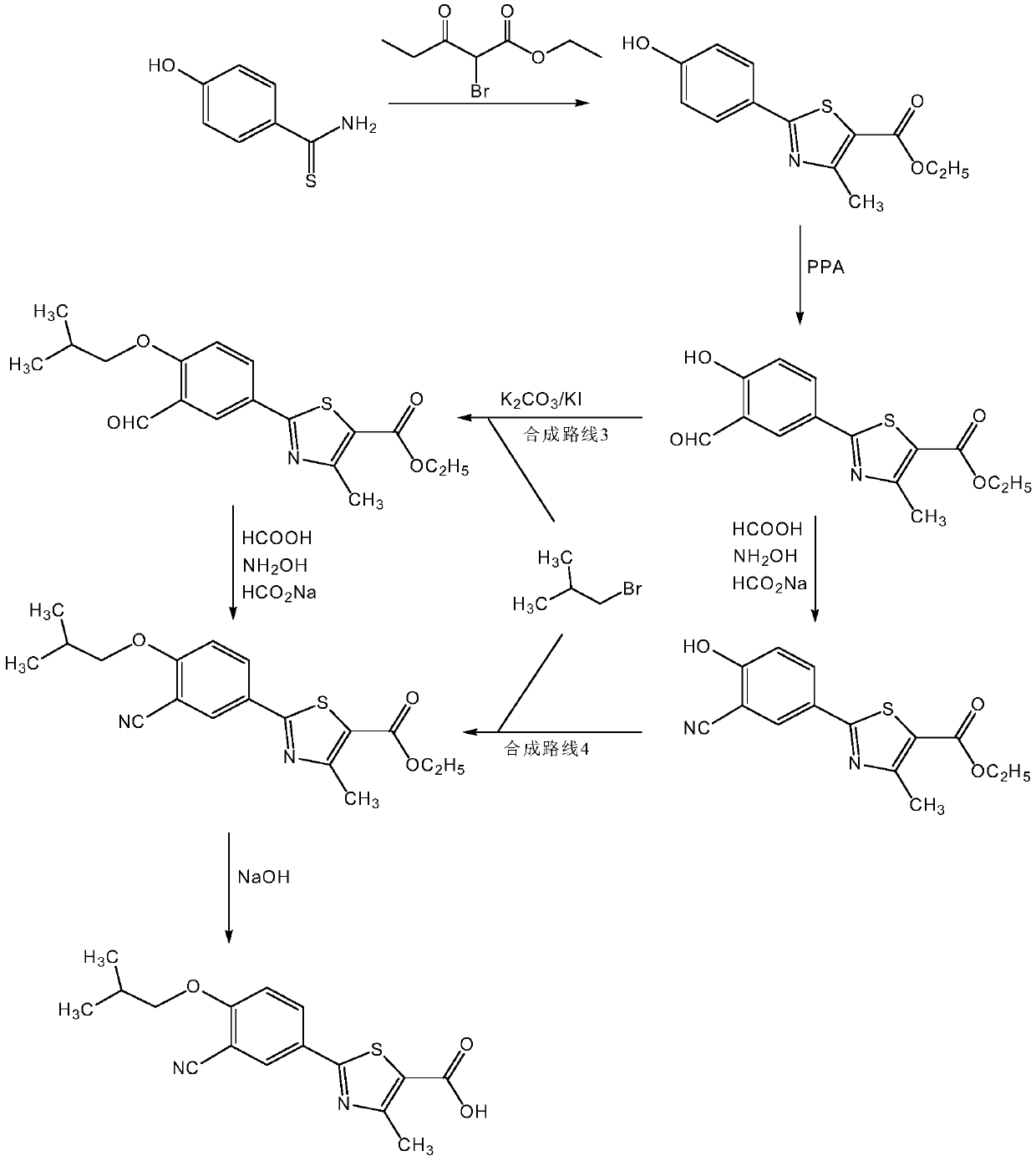
[0109] Example 1: Preparation of ethyl 2-(3-formaldehyde-4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate
[0110] (1) Add 1 molar portion of the reactant 2-(4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylic acid ethyl ester (actual dosage 80g) to (in a 1L reaction bottle) and preheat to 80 In a homogeneous mixture composed of 1.75 molar parts of polyphosphoric acid and 2.5 molar parts of methanesulfonic acid at ℃, stir evenly;
[0111] (2) Add 1.4 molar parts of Duff reaction reagent hexamethylenetetramine to the above reaction mixture under stirring, continue to react at a temperature of 80° C. for 6 hours, and cool the reaction mixture to 40° C. after the reaction is completed;
[0112] (3) Slowly add saturated ice brine (to about 80% of the total capacity of the reaction flask) into the cooled reaction mixture, filter after the solid is precipitated, wash the solid with water to neutrality, and dry to obtain a light yellow solid as 2- (3-formyl-4-hydroxyphenyl)-4-methyl-5-th...
Embodiment 2
[0113] Example 2: Preparation of ethyl 2-(3-formaldehyde-4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate
[0114] (1) Add 1 molar portion of reactant 2-(4-hydroxyphenyl)-4-methyl-5-thiazole formic acid ethyl ester (actual feeding amount 80g) to (in 1L reaction bottle) and preheat to 70 In a homogeneous mixture composed of 3 molar parts of polyphosphoric acid and 2 molar parts of methanesulfonic acid at ℃, stir evenly;
[0115] (2) Add 1.5 molar parts of Duff reaction reagent hexamethylenetetramine to the above reaction mixture under stirring, continue to react at a temperature of 70° C. for 8 hours, and cool the reaction mixture to 37° C. after the reaction is completed;
[0116] (3) Slowly add saturated ice brine (to about 80% of the total capacity of the reaction flask) into the cooled reaction mixture, filter after the solid is precipitated, wash the solid with water to neutrality, and dry to obtain a light yellow solid as 2- Ethyl (3-formaldehyde-4-hydroxyphenyl)-4-meth...
Embodiment 3
[0117] Example 3: Preparation of ethyl 2-(3-formaldehyde-4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate
[0118] (1) Add 1 molar portion of the reactant 2-(4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylic acid ethyl ester (actual dosage 80g) to (in a 1L reaction bottle) and preheat to 100 In a homogeneous mixture composed of 1.5 molar parts of polyphosphoric acid and 2 molar parts of methanesulfonic acid at ℃, stir evenly;
[0119] (2) Add 1.2 molar parts of Duff reaction reagent hexamethylenetetramine to the above reaction mixture under stirring, continue to react for 7 hours at a temperature of 75°C, and cool the reaction mixture to 38°C after the reaction is completed;
[0120] (3) Slowly add saturated ice brine (to about 80% of the total capacity of the reaction flask) into the cooled reaction mixture, filter after the solid is precipitated, wash the solid with water to neutrality, and dry to obtain a light yellow solid as 2- Ethyl (3-formaldehyde-4-hydroxyphenyl)-4-meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com