Preparation method of compound with anti-tumor effect
A technology of anti-tumor effect and compound, applied in the direction of organic chemistry, can solve the problems of low degree of purification, complicated process of artificial synthesis of urolithin, difficulty in industrialized large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
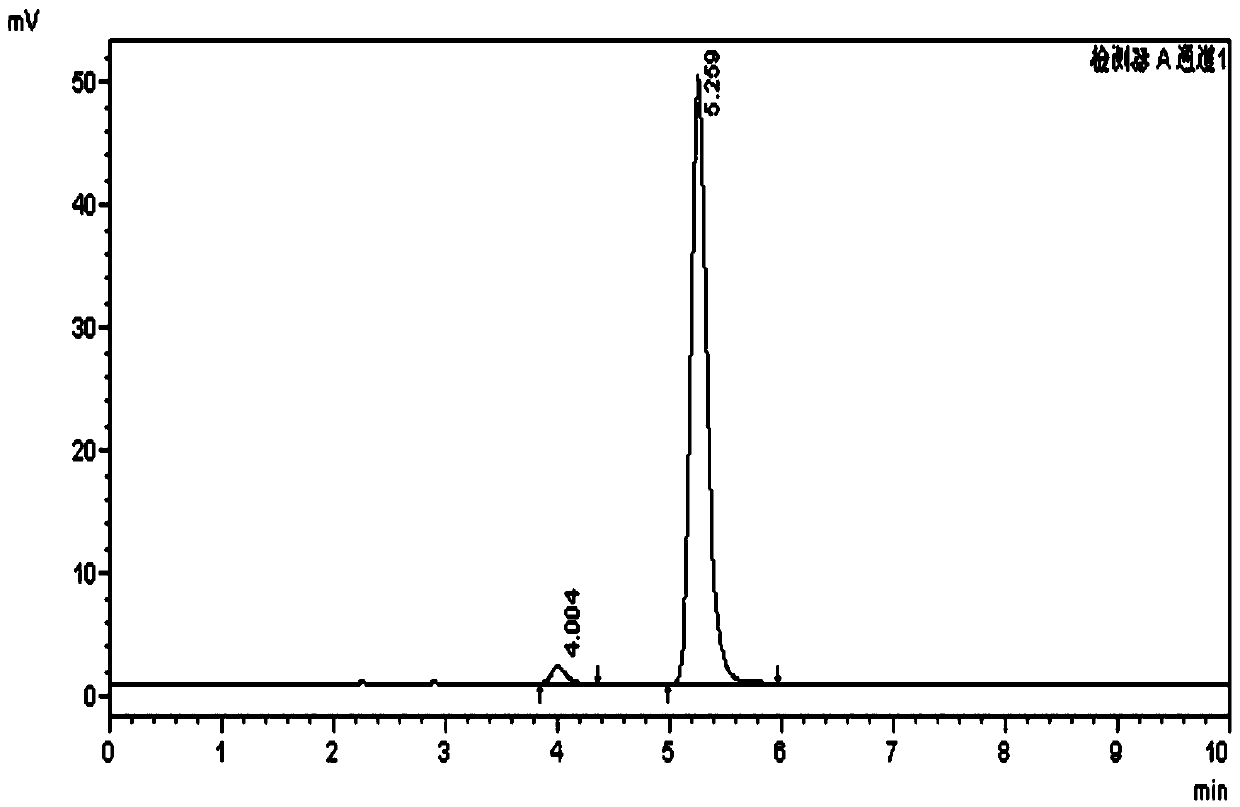
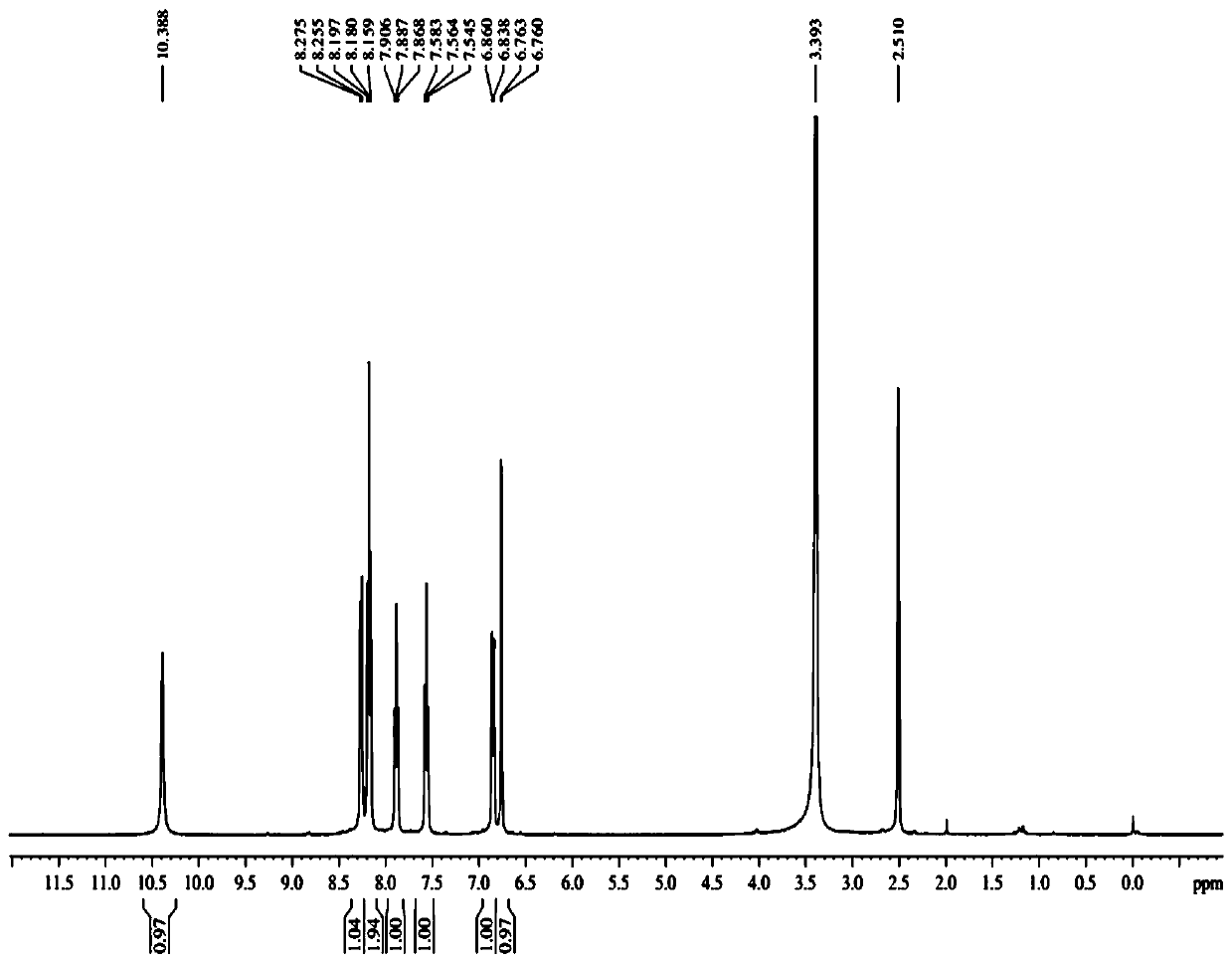
Image
Examples
Embodiment 1
[0039] The preparation method of urolithin B comprises the following steps:
[0040] (1) Add and dissolve a certain amount of sodium hydroxide in deionized water, the mass ratio is sodium hydroxide: deionized water = 1:10, stir evenly, fully dissolve, and obtain sodium hydroxide solution, which is set aside;
[0041] (2) Pour the sodium hydroxide solution obtained in step (1) into a three-necked flask containing a certain amount of o-bromobenzoic acid, heat the three-necked flask with a water bath, keep the temperature at 50°C, and use mechanical stirring at a speed of 200r / min, after the o-bromobenzoic acid is completely dissolved, add a certain amount of resorcinol and continue heating until the color no longer darkens. Wherein the weight of o-bromobenzoic acid is 2 times of the solid weight of sodium hydroxide, and the weight ratio of o-bromobenzoic acid and resorcinol is o-bromobenzoic acid: resorcinol=1:1;
[0042] (3) Add copper sulfate solution with a mass concentrati...
Embodiment 2
[0050] The preparation method of urolithin B comprises the following steps:
[0051] (1) Add and dissolve a certain amount of sodium hydroxide in deionized water, the mass ratio is sodium hydroxide: deionized water = 1:10, stir evenly, fully dissolve, and obtain sodium hydroxide solution, which is set aside;
[0052] (2) Pour the sodium hydroxide solution obtained in step (1) into a three-necked flask containing a certain amount of o-bromobenzoic acid, heat the three-necked flask with a water bath, keep the temperature at 80°C, use mechanical stirring, and rotate at a speed of 800r / min, after the o-bromobenzoic acid is completely dissolved, add a certain amount of resorcinol and continue heating until the color no longer darkens. Wherein the weight of o-bromobenzoic acid is 3 times of sodium hydroxide solid weight, and the weight ratio of o-bromobenzoic acid and resorcinol is: o-bromobenzoic acid: resorcinol=1:1;
[0053] (3) Add copper sulfate solution with a mass concentra...
Embodiment 3
[0061] The preparation method of urolithin B comprises the following steps:
[0062] (1) Add and dissolve a certain amount of sodium hydroxide in deionized water, the mass ratio is sodium hydroxide: deionized water = 1:10, stir evenly, fully dissolve, and obtain sodium hydroxide solution, which is set aside;
[0063] (2) Pour the sodium hydroxide solution obtained in step (1) into a three-necked flask containing a certain amount of o-bromobenzoic acid, and heat the three-necked flask with a water bath to keep the temperature at 60°C. Use mechanical stirring at a speed of 500r / min, after the o-bromobenzoic acid is completely dissolved, add a certain amount of resorcinol and continue heating until the color no longer darkens. Wherein the weight of o-bromobenzoic acid is 3 times of sodium hydroxide solid weight, and the weight ratio of o-bromobenzoic acid and resorcinol is: o-bromobenzoic acid: resorcinol=1:1;
[0064] (3) Add copper sulfate solution with a mass concentration o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com